��Ŀ����
8���������ڱ��е�һ���֣�����A-I�����ڱ��е�λ�ã���Ԫ�ط��ϻ�ѧʽ�ش��������⣺| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | |||||||
| 2 | D | E | G | |||||
| 3 | B | C | J | F | H | I |
��2��B�ļ������ӽṹʾ��ͼΪ
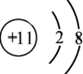 ��
����3����B��C��D��E��F�У�ԭ�Ӱ뾶������Na��
��4��A�ֱ���D��E��G�γɵĻ������У����ȶ���HF��
��5������������ˮ���������ǿ����NaOH��������ǿ����HClO4�������Ե���Al��OH��3��
���� ��Ԫ�������ڱ���λ�ã���֪AΪ�⡢BΪNa��CΪAl��DΪ̼��EΪ����FΪ�ס�GΪ����HΪCl��IΪAr��JΪSi��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ����ѧ��������ã��������ư뵼����ϵ�Ԫ���ǹ裻
��2��B�ļ�������ΪNa+�����������Ϊ10�����ݺ�������Ų�������д��
��3��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����һ����Ӳ�Խ��ԭ�Ӱ뾶Խ��
��4���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���
��5���ƵĽ�������ǿ�����������Ƶļ�����ǿ��������ǿ���Ǹ����ᣬ�����������������������
��� �⣺��Ԫ�������ڱ���λ�ã���֪AΪ�⡢BΪNa��CΪAl��DΪ̼��EΪ����FΪ�ס�GΪ����HΪCl��IΪAr��JΪSi��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��Ar�Ļ�ѧ��������ã��������ư뵼����ϵ�Ԫ���ǹ裬
�ʴ�Ϊ��Ar��Si��
��2��B�ļ�������ΪNa+�����������Ϊ10����������Ų����ɣ����ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��3��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����һ����Ӳ�Խ��ԭ�Ӱ뾶Խ��ԭ�Ӱ뾶Na��Al��Si��C��N��
�ʴ�Ϊ��Na��
��4��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����HF���ȶ���
�ʴ�Ϊ��HF��
��5���ƵĽ�������ǿ����NaOH�ļ�����ǿ��������ǿ����HClO4��Al��OH��3���������������
�ʴ�Ϊ��NaOH��HClO4��Al��OH��3��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ����ã���������Ԫ�����ڱ��Ľṹ�������Ԫ�أ��ӽṹ������ͬ���ڡ�ͬ����Ԫ�����ʵݱ���ɣ�

| A�� | 60s����XŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.001 mol/��L•s�� | |
| B�� | �����������Ϊ20L��Z��ƽ��Ũ�ȱ�Ϊԭ����$\frac{1}{2}$ | |
| C�� | ������ѹǿ��������Y��ת���ʼ�С | |
| D�� | �������¶ȣ�X���������������÷�Ӧ������ӦΪ���ȷ�Ӧ |
| A�� | NaOH | B�� | Ca��OH��2 | C�� | Mg��OH��2 | D�� | Al��OH��3 |
| A�� | NaOH�����壩 | B�� | H2O | C�� | NH4Cl�����壩 | D�� | CH3COOH |
| A�� | �����ơ�NaNO2���� | B�� | ��������FeO�������� | ||
| C�� | ���Na2CO3���� | D�� | �ƾ���CH3CH2OH���л��� |
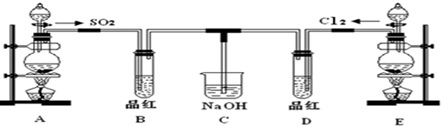
 �״���һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ����
�״���һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ����