��Ŀ����
1�� Ni��La�ĺϽ���Ŀǰʹ�ù㷺�Ĵ�����ϣ��úϽ�ľ����ṹ��ͼ��ʾ��
Ni��La�ĺϽ���Ŀǰʹ�ù㷺�Ĵ�����ϣ��úϽ�ľ����ṹ��ͼ��ʾ���ٸþ���Ļ�ѧʽΪLaNi5��
����֪�þ�����Ħ������ΪM g•mol-1���ܶ�Ϊd g•cm-3���þ����������$\frac{M}{N{\;}_{A}d}$cm3���ú�M��d��NA�Ĵ���ʽ��ʾ����
�۴�����ϵ��о��Ƿ�չ����Դ�ļ����ѵ�֮һ��ij���ʵķ��ӿ���ͨ������γɡ���״�ṹ�������ɳ�ΪDZ�ڴ�����ϣ���÷���һ����������BC��
A��H2O B��CH4 C��HF D��CO��NH2��2��
���� �ٸ��ݾ�̯�����㾧����ʵ�ʺ��е�ԭ�Ӹ�����ȷ����ѧʽ��
�ڸ���һ������������m=��v���㣻
��CH4���Ӽ�û�������HF���Ӽ�ֻ���γ���״�ṹ��
��� �⣺�ٸúϽ�ľ�����ͼ��ʾ������������һ����ԭ�ӣ�����8����ԭ�Ӷ��ھ������ϣ���ԭ�Ӷ��ھ������㣮
���Ծ���ʵ�ʺ��е���ԭ��Ϊ1��1+$\frac{1}{2}$��8=5������ʵ�ʺ��е���ԭ��Ϊ8��$\frac{1}{8}$=1�����Ծ���Ļ�ѧʽLaNi5���ʴ�Ϊ��LaNi5��
��һ������������m=$\frac{M}{N{\;}_{A}}$������m=��v����v=$\frac{M}{N{\;}_{A}d}$��
�ʴ�Ϊ��$\frac{M}{N{\;}_{A}d}$��
��CH4���Ӽ�û����������γɡ���״�ṹ����ÿ��HFֻ���γ�2�����������HF���Ӽ�ֻ���γ���״�ṹ����ѡBC��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢�����֪ʶ�㣬���ؿ�������������ռ�������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
12�����淴Ӧ��2NO2��g��?2NO��g��+O2��g��������̶����ܱ������н��У��ﵽƽ��״̬�ı�־���ǣ�������
�ٵ�λʱ��������n mol O2��ͬʱ����2n mol NO2��
�ڻ��������ܶȲ��ٸı��״̬
�ۻ�������ƽ����Է����������ٸı��״̬
�ܵ�λʱ��������n mol O2��ͬʱ����2n mol NO
�ݻ�������ѹǿ���ٸı��״̬��
�ٵ�λʱ��������n mol O2��ͬʱ����2n mol NO2��
�ڻ��������ܶȲ��ٸı��״̬
�ۻ�������ƽ����Է����������ٸı��״̬
�ܵ�λʱ��������n mol O2��ͬʱ����2n mol NO
�ݻ�������ѹǿ���ٸı��״̬��
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | ȫ�� |
6���������ڹ���Ԫ��Mn��Fe��Ti��Ni����C��H��O�γɶ��ֻ����
��1������������ȷ����AD��������ĸ��
A��CH2O��ˮ���Ӽ����γ����
B��CH2O��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�����к���6���Ҽ���1����м���C6H6�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
��2��Mn��Fe�IJ��ֵ������������±���
Mnԭ�Ӽ۵����Ų�ʽΪ3d54s2����̬Mn2+��ʧȥһ�����ӱ���̬Fe2+��ʧȥһ�������ѣ���ԭ������Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶࣬��Fe2+��Fe3+ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�
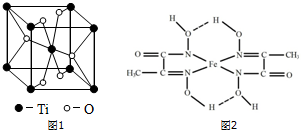
��3������Ԫ��ԭ�ӵ���Χ�����Ų����ɽ�Ԫ�����ڱ�����������Ti����d����Ti��һ��������X���侧���ṹ��ͼ1��ʾ����X�Ļ�ѧʽΪTiO2��
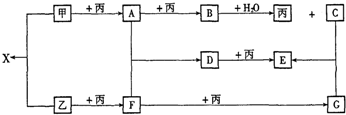
��4��ij���Ļ�����ṹ��ʽ��ͼ2��ʾ
����������������и��ǽ���Ԫ�ص縺���ɴ�С��˳��ΪO��N��C��H ����Ԫ�ط��ű�ʾ��
����ͼ2���á���������������ӵ���λ����
��5��NiO��������������Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2-����λ��Ϊ6���⼸��O2-���ɵĿռ乹��Ϊ�������壮��֪Ni2+��O2-�ĺ˼��Ϊanm��NiO��Ħ������ΪM g/mol�������ӵ�������NA��ʾ����þ�����ܶ�Ϊ$\frac{M��1{0}^{21}}{2{a}^{3}{N}_{A}}$ g/cm3��
��1������������ȷ����AD��������ĸ��
A��CH2O��ˮ���Ӽ����γ����
B��CH2O��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�����к���6���Ҽ���1����м���C6H6�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
��2��Mn��Fe�IJ��ֵ������������±���
| Ԫ �� | Mn | Fe | |
| ������ /kJ•mol-1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
��3������Ԫ��ԭ�ӵ���Χ�����Ų����ɽ�Ԫ�����ڱ�����������Ti����d����Ti��һ��������X���侧���ṹ��ͼ1��ʾ����X�Ļ�ѧʽΪTiO2��

��4��ij���Ļ�����ṹ��ʽ��ͼ2��ʾ
����������������и��ǽ���Ԫ�ص縺���ɴ�С��˳��ΪO��N��C��H ����Ԫ�ط��ű�ʾ��
����ͼ2���á���������������ӵ���λ����
��5��NiO��������������Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2-����λ��Ϊ6���⼸��O2-���ɵĿռ乹��Ϊ�������壮��֪Ni2+��O2-�ĺ˼��Ϊanm��NiO��Ħ������ΪM g/mol�������ӵ�������NA��ʾ����þ�����ܶ�Ϊ$\frac{M��1{0}^{21}}{2{a}^{3}{N}_{A}}$ g/cm3��
15������C��CH3��3CH2CH3˵���д�����ǣ�������
| A�� | ����������2��2-�������� | |
| B�� | ���ĺ˴Ź���������3���� | |
| C�� | ���ķ���ʽΪC6H14 | |
| D�� | ������ϩ����H2�ӳɶ�������ϩ�������� |
16����pH=1����ɫ��Һ�У����и�����������������ԭ��Ӧ�����ܹ�����ǣ�������
| A�� | NH4+ K+ Na+ CO32- NO3- | B�� | K+ Na+ Fe2+ SO42- NO3- | ||
| C�� | NH4+ K+ Na+ HCO3-Cl- | D�� | NH4+ K+ Na+ NO3- I- |
 ��GSO3��
��GSO3�� ��
�� ��
��