��Ŀ����
9����Ȼ��ij����F�ĺϳ�·�����£����ֲ�����ȥ����
��֪��
��1��

��2��

��ش��������⣺
��1���Լ�b�й����ŵ�������̼̼˫������ԭ�ӣ�A��ϵͳ������2-��-1��3-����ϩ��A��B�ķ�Ӧ������ȡ����Ӧ��
��2���Լ�a�Ľṹ��ʽ��HCHO��E�Ľṹ��ʽ��
 ��
����3���Լ�b��NaOH��Һ���ȵĻ�ѧ����ʽ��
 ��
����4��C��������Һ��Ӧ�Ļ�ѧ����ʽ��
 ��
����5����������������D��ͬ���칹��Ľṹ��ʽ
 ��
������ʹ��ˮ��ɫ������ʹ������Ȼ�̼��Һ��ɫ ���ú�����ײ�÷����о���
 �ṹ��
�ṹ��
���� A�����Ӿ۷�Ӧ���ɸ߷��ӻ�������ݸ߷��ӻ�����ṹ��ʽ֪AΪCH2=C��CH3��-CH=CH2���Ƚ� CH2=C��CH3����A�Ľṹ��������Ϣ��1����֪�Լ�aΪHCHO��A���Լ�b����ȡ����Ӧ����B���Ƚ�B��F�Ľṹ��֪��EΪ ��D�ͼ״�����������Ӧ����E����DΪCH2=C��CH3��-COOH��C������D����CΪCH2=C��CH3��-CHO��CH2=C��CH3��������C���ݴ˷������
��D�ͼ״�����������Ӧ����E����DΪCH2=C��CH3��-COOH��C������D����CΪCH2=C��CH3��-CHO��CH2=C��CH3��������C���ݴ˷������
��� �⣺A�����Ӿ۷�Ӧ���ɸ߷��ӻ�������ݸ߷��ӻ�����ṹ��ʽ֪AΪCH2=C��CH3��-CH=CH2���Ƚ� CH2=C��CH3����A�Ľṹ��������Ϣ��1����֪�Լ�aΪHCHO��A���Լ�b����ȡ����Ӧ����B���Ƚ�B��F�Ľṹ��֪��EΪ ��D�ͼ״�����������Ӧ����E����DΪCH2=C��CH3��-COOH��C������D����CΪCH2=C��CH3��-CHO��CH2=C��CH3��������C��
��D�ͼ״�����������Ӧ����E����DΪCH2=C��CH3��-COOH��C������D����CΪCH2=C��CH3��-CHO��CH2=C��CH3��������C��
��1��b�й�����Ϊ̼̼˫������ԭ�ӣ�A��������2-��-1��3-����ϩ��A����ȡ����Ӧ����B��
�ʴ�Ϊ��̼̼˫������ԭ�ӣ� 2-��-1��3-����ϩ��ȡ����Ӧ��
��2��ͨ�����Ϸ���֪��aΪHCHO��E�ṹ��ʽΪ ��
��
�ʴ�Ϊ��HCHO�� ��
��
��3���Լ�b��NaOH��Һ���ȵĻ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��C����������Ӧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��D��ͬ���칹���������������
����ʹ��ˮ��ɫ������ʹ������Ȼ�̼��Һ��ɫ��˵������ȩ����
���ú�����ײ�÷����о��� �ṹ��
�ṹ��
�����������ͬ���칹��Ϊ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ���۲�����ж����������ݷ�Ӧǰ�����ʽṹ�仯ȷ����Ӧ���ͼ�ijЩ��Ӧ���ȷ�ƶ����ʽṹ��ʽ�ǽⱾ��ؼ����������ճ����л���Ӧ���͡���Ӧ����������ʽ����д����Ŀ�ѶȲ���

| A�� | �����ᴿ | B�� | ������� | C�� | ��������ˮ | D�� | ���ˮ |
| A�� | Ca��HCO3��2��aq����Ca��OH��2��aq�� | B�� | Ca��HCO3��2��aq����NaOH��aq�� | ||
| C�� | NaHCO3 ��aq����Ca��OH��2��aq�� | D�� | Mg��HCO3��2��aq����NaOH ��aq�� |
| A�� | 0.1mol�Ա��������к���˫������ĿΪ0.2NA | |
| B�� | �����£�1L pH=13��Ba��OH��2��Һ�к���OH-����ĿΪ0.2NA | |
| C�� | Fe������Cl2��ȼ������0.5 mol���ת�Ƶĵ�����ΪNA | |
| D�� | ��״���£�2.24L H2Sȫ������ˮ������Һ��HS-��S2-������֮��Ϊ0.1NA |

| A�� | Һ��Ӧʢ������ˮ�ܷ����ò�������������ɫ�Լ�ƿ�� | |
| B�� | ��ʪ��ĺ�ɫʯ����ֽ������������ | |
| C�� | ��װ�ü��Ʊ����� | |
| D�� | ��װ���Ҽ��ȷֽ�Al��OH��3���� |
| A�� | 3g H2 | B�� | 11.2L HCl | C�� | 1.12L H2O | D�� | 3.01��1023��CH4 |
| A�� | ����������ԭ��Ӧ | B�� | ��˿�ӵ�Դ�ĸ��� | ||
| C�� | ��������������Һ�������� | D�� | ���������������� |
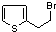 ��
�� ��
��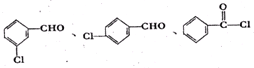 ��
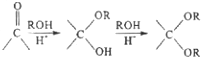
��
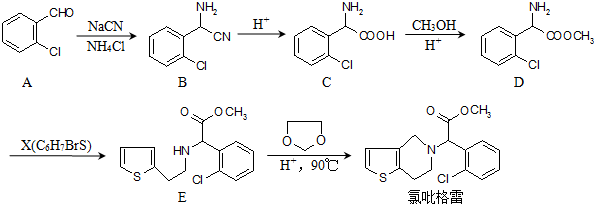
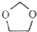 �ĺϳ�·��ͼ�����Լ���ѡ��
�ĺϳ�·��ͼ�����Լ���ѡ��
