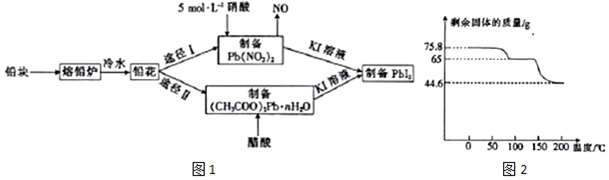
��Ŀ����
16������18.4mol/L��Ũ�������Ƴ�Ũ��Ϊ0.5mol•L-1��ϡ����250ml����1����ʵ��ʹ�õ��������У�������ƽ��ҩ�ף���Ͳ�����������Լ�ƿ����ȱ�ٵ������� ��250mL����ƿ���ڽ�ͷ�ιܣ����ձ���
��2������Ũ��������Ϊ6.8mL
��3���뽫���и�����������ȷ��������ں����ϣ�
A������Ͳ��ȡŨH2SO4 B�������ߵ�ҡ�� C���ý�ͷ�ιܼ�����ˮ���̶��� D������Һת������ƿE��ϡ��ŨH2SO4 �������ȷ��˳������ΪAEDCB
��4�������������ʹ������ҺŨ����ƫ��ã���ƫ�ߡ�����ƫ�͡�������Ӱ�족��
��д���пհף�
��Ũ����ϡ�ͺ�ֱ��ת������ƿ�����ݣ���ʹŨ��ƫ�ߣ�
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ�������ˮ���̶���ƫ�ͣ�
������Ͳ��ȡŨ������������Ͳ��ʹŨ��ƫ�ͣ���Һ����ʱ��������ƿ��ʹŨ��ƫ�ͣ�
���� ��1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������з�����
��2��������Һϡ������������������ʵ�������������ҪŨ����������
��3����������һ�����ʵ�����Ũ����Һ�ķ�����������
��4������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1����18.4mol/L��Ũ�������Ƴ�Ũ��Ϊ0.5mol•L-1��ϡ����250ml��ʹ�õ���������Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ����Ի���Ҫ������Ϊ��250mL����ƿ����ͷ�ιܡ��ձ���
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ��ձ���
��2������ҪŨ��������ΪV����������Һϡ������������������ʵ�������ã�18.4mol/L��V=0.5mol•L-1��250mL�����V=6.8mL��
�ʴ�Ϊ��6.8��
��3�����Ʋ���Ϊ����ȡŨ���ᡢŨ�����ϡ�͡�ת�ơ����ݡ�ҡ�ȡ�ת���Լ�ƿ��������ȷ˳��Ϊ��AEDCB��
�ʴ�Ϊ��AEDCB��
��4����Ũ����ϡ�ͺ�ֱ��ת������ƿ�����ݣ�Ũ����ϡ��ʱ�����������ȣ���ȴ��Һ���½���������Һ���ƫС����ʹŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ�������ˮ���̶��ߵ�����Һ���ƫ��ʹ��ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
������Ͳ��ȡŨ������������Ͳ������ȡ��Ũ�������ƫС����������ʵ���ƫС����ʹ��ҺŨ��ƫ�ͣ���Һ����ʱ��������ƿ������Һ���ƫ����ʹŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ�
���� ���⿼��һ�����ʵ���Ũ����Һ���ƹ��̡����ʵ���Ũ���йؼ�����������ȣ���ȷ����ԭ�������������ǽ���ؼ����ѶȲ���������Ϊ�״��㣮

| A�� | 8 | B�� | 13 | C�� | 14 | D�� | 18 |
| A�� | �ڿ��������ձ���CO2�������ڿ�����Ⱦָ�� | |
| B�� | �ճ������к�ҽԺ������ˮ�Ҵ�ɱ������ | |
| C�� | ��ɫʳƷ��ָ�����κλ�ѧ���ʵ�ʳƷ | |
| D�� | Ŀǰ�ӵ�ʳ������Ҫ���ӵ���KIO3 |
��1��LiCoO2����Ԫ�صĻ��ϼ�Ϊ+3��
��2����ҵ�Ͻ���﮵�ص��������������������Һ��ϡ�����ϼ��ȣ��ɵõ�CoSO4���գ���Ӧ�Ļ�ѧ����ʽΪ2LiCoO2+H2O2+3H2SO4=Li2SO4+2CoSO4+O2+4H2O�����������H2SO4��H2O2�Ļ��Һ����ȱ�������ɵ����������Ⱦ���������ӷ����˷Ѻܴ�
������һ�ֺ��ܿ�ʯ[��Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��]��ȡCoC2O4•2H2O�����������£�
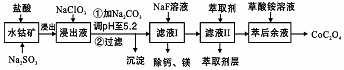
��֪����֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH������
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ȫ������pH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��4������ƽ���ƶ�ԭ��˵����Na2CO3��PH��5.2���ɳ�����ԭ����Fe3+��Al3+����M3+���棩��ˮ��Һ�д���ƽ��M3++H2O?M��OH��3+3H+������̼���ƺ�CO32-��H+��������ѵ����HCO3-��ʹˮ��ƽ�����ƶ�����������
��5����Һ���м�����ȡ���������dz�ȥMn2+��
��6�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪ij�¶��£�Ksp��MgF2��=7.35��10-11��Ksp��CaF2��=1.05��10-10�����������NaF��������Һc��Mg2+��/c��Ca2+��=0.7��
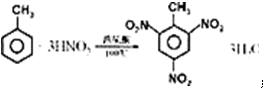 ��
��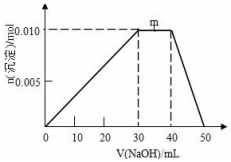 ��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£�
��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£�