��Ŀ����
��úΪ��Ҫԭ�Ͽ����Ʊ��Ҷ�������ع����������£�

��1��д������l�ڴ�����������ֱ����ȡ�Ҷ����Ļ�ѧ����ʽ
��2���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʣ��������ʽ��úϳ���Ϊԭ�Ͼ��ܵõ���ԭ��������Ϊ100%���� ������ĸ����
A�����ᣨ HOOC-COOH�� B���״���CH3OH�� C������[CO��NH2��2]
��3����ҵ�ϻ�����������Ȼ������Ҫ�ɷ�ΪCH������C02��Ӧ�Ʊ��ϳ�������֪��
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ/mol
2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol
2CO��g��+O2��g���T2CO2��g����H=-566��kJ/mol
��CH4��CO2���ɺϳ������Ȼ�ѧ����ʽΪ ��
��4������2���ں����ܱ�������Ͷ������������H2�������·�Ӧ��CH3OOC-COOCH3��g��+4H2��g��?HOCH2CH2OH��g��+2CH3OH��g����H=-34kJ/mol
Ϊ����Ҷ����IJ��������ʣ��˲��õĴ�ʩ�� ������ĸ����
A�������¶� B������ѹǿ C����������Ũ��
��5�����������ˮ�����ɲ��CH3OOC-COOCH3=2H2O?2CH3OH+HOOC-COOH
�ٲ����Ƕ�Ԫ���ᣬ�����Ʊ�KHC2O4 ��������أ���KHC2O4��Һ�����ԣ��û�ѧƽ��ԭ�����ͣ� ��
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ����й�ϵһ������ȷ����
������ĸ����
A��c��Na+����c��K+����c��C2O42-����c��HC2O4-��
B��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4��
C��c��K+��+c��Na+��=c��HC2O4-��+c��C2O42-��
��6���Ҷ�����������KOH��Һ�й���ȼ�ϵ�أ������Ҷ����ĵ缫Ϊ��Դ�� �����������������������ӦʽΪ ��

��1��д������l�ڴ�����������ֱ����ȡ�Ҷ����Ļ�ѧ����ʽ
��2���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʣ��������ʽ��úϳ���Ϊԭ�Ͼ��ܵõ���ԭ��������Ϊ100%����
A�����ᣨ HOOC-COOH�� B���״���CH3OH�� C������[CO��NH2��2]
��3����ҵ�ϻ�����������Ȼ������Ҫ�ɷ�ΪCH������C02��Ӧ�Ʊ��ϳ�������֪��
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ/mol
2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol
2CO��g��+O2��g���T2CO2��g����H=-566��kJ/mol
��CH4��CO2���ɺϳ������Ȼ�ѧ����ʽΪ
��4������2���ں����ܱ�������Ͷ������������H2�������·�Ӧ��CH3OOC-COOCH3��g��+4H2��g��?HOCH2CH2OH��g��+2CH3OH��g����H=-34kJ/mol
Ϊ����Ҷ����IJ��������ʣ��˲��õĴ�ʩ��
A�������¶� B������ѹǿ C����������Ũ��
��5�����������ˮ�����ɲ��CH3OOC-COOCH3=2H2O?2CH3OH+HOOC-COOH
�ٲ����Ƕ�Ԫ���ᣬ�����Ʊ�KHC2O4 ��������أ���KHC2O4��Һ�����ԣ��û�ѧƽ��ԭ�����ͣ�
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ����й�ϵһ������ȷ����
A��c��Na+����c��K+����c��C2O42-����c��HC2O4-��
B��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4��
C��c��K+��+c��Na+��=c��HC2O4-��+c��C2O42-��
��6���Ҷ�����������KOH��Һ�й���ȼ�ϵ�أ������Ҷ����ĵ缫Ϊ��Դ��
���㣺�Ȼ�ѧ����ʽ,��ѧ��Դ���͵��,��ѧƽ���Ӱ������,����Ũ�ȴ�С�ıȽ�
ר�⣺
��������1�����ݷ�Ӧ����������Ϸ�Ӧ�ص���д��ѧ����ʽ��
��2�����������غ㶨����������
��3�����ݸ�˹������������
��4��ͨ������ƽ����ƶ����жϣ�
��5����HC2O4-����ˮ�����ܵ��룬�ݴ˷�����
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�����Һ�е�����Ϊ��KHC2O4��K2C2O4����������������غ�͵���غ���������
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磬��ϵ������Һ�Ļ�������д������Ӧ��
��2�����������غ㶨����������
��3�����ݸ�˹������������
��4��ͨ������ƽ����ƶ����жϣ�
��5����HC2O4-����ˮ�����ܵ��룬�ݴ˷�����
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�����Һ�е�����Ϊ��KHC2O4��K2C2O4����������������غ�͵���غ���������
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磬��ϵ������Һ�Ļ�������д������Ӧ��
���
�⣺��1���ϳ�������Ҫ�ɷ�ΪCO��H2���ڴ��������ºϳ��Ҷ����ķ�Ӧ��2CO+3H2
HOCH2CH2OH���ʴ�Ϊ��2CO+3H2
HOCH2CH2OH��
��2�����������غ㶨�ɿ�֪���ϳ�������Ҫ�ɷ�ΪCO��H2��������Ԫ�أ��ʲ����ܺϳɳ����أ���C��ѡ������A�Ҷ����У�C��Oԭ�Ӹ�����Ϊ1��2��������CO�е�1��1���ʲ����ɺϳ������ϳɣ���B���״���CH3OH�� ������CO��H2��1��2���ϳɣ�ȫ��ԭ�Ӿ�ת��ΪĿ����ԭ��ת���ʴﵽ��100%����ѡB��
��3����֪��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ/mol ��
2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol ��
2CO��g��+O2��g���T2CO2��g����H=-566��kJ/mol ��
����-��-�ۿɵã�
CH4��g��+CO2��g��=2CO��g��+2H2��g����H=��-890.3kJ/mol��-��-571.6kJ/mol��-��-566��kJ/mol��=+247.3KJ/mol���ʴ�Ϊ��CH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247.3KJ/mol��
��4��A�������¶ȣ�ƽ�����ƣ��Ҷ����IJ������ͣ���A��ѡ��
B������ѹǿ����Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Bѡ��
C����������Ũ�ȣ���Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Cѡ��
��ѡBC��
��5����HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-�����������ԣ�HC2O4-+H2O?H2C2O4+OH-��ˮ���Լ��ԣ���HHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ��������ˮ�⣬�ʴ�Ϊ��HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-����HC2O4-�ĵ��������ˮ�⣮
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�������NaOH����������٣�������KHC2O4��Ӧ��������Һ�е�����Ϊ��KHC2O4��K2C2O4��Na2C2O4��
A�����ڼ����NaOH����������٣�������KHC2O4��Ӧ������c��Na+�������ܴ���c��K+������A����
B����Һ�е�K+��HC2O4-��H2C2O4��C2O42-��������KHC2O4�����������غ��֪��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4������B��ȷ��
C�����ݵ���غ��֪��c��K+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-������C����
��ѡB��
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磻�����Ǽ���ȼ�ϵ�أ��ʸ����Ҷ����ŵ�����CO32-���缫����ʽΪ��
HOCH2CH2OH-10e-+14OH-=2CO32-+10H2O���ʴ�Ϊ������HOCH2CH2OH-10e-+14OH-=2CO32-+10H2O��
| ||
| ||
��2�����������غ㶨�ɿ�֪���ϳ�������Ҫ�ɷ�ΪCO��H2��������Ԫ�أ��ʲ����ܺϳɳ����أ���C��ѡ������A�Ҷ����У�C��Oԭ�Ӹ�����Ϊ1��2��������CO�е�1��1���ʲ����ɺϳ������ϳɣ���B���״���CH3OH�� ������CO��H2��1��2���ϳɣ�ȫ��ԭ�Ӿ�ת��ΪĿ����ԭ��ת���ʴﵽ��100%����ѡB��
��3����֪��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ/mol ��
2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol ��
2CO��g��+O2��g���T2CO2��g����H=-566��kJ/mol ��
����-��-�ۿɵã�
CH4��g��+CO2��g��=2CO��g��+2H2��g����H=��-890.3kJ/mol��-��-571.6kJ/mol��-��-566��kJ/mol��=+247.3KJ/mol���ʴ�Ϊ��CH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247.3KJ/mol��
��4��A�������¶ȣ�ƽ�����ƣ��Ҷ����IJ������ͣ���A��ѡ��
B������ѹǿ����Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Bѡ��
C����������Ũ�ȣ���Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Cѡ��
��ѡBC��
��5����HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-�����������ԣ�HC2O4-+H2O?H2C2O4+OH-��ˮ���Լ��ԣ���HHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ��������ˮ�⣬�ʴ�Ϊ��HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-����HC2O4-�ĵ��������ˮ�⣮
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�������NaOH����������٣�������KHC2O4��Ӧ��������Һ�е�����Ϊ��KHC2O4��K2C2O4��Na2C2O4��
A�����ڼ����NaOH����������٣�������KHC2O4��Ӧ������c��Na+�������ܴ���c��K+������A����
B����Һ�е�K+��HC2O4-��H2C2O4��C2O42-��������KHC2O4�����������غ��֪��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4������B��ȷ��
C�����ݵ���غ��֪��c��K+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-������C����
��ѡB��
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磻�����Ǽ���ȼ�ϵ�أ��ʸ����Ҷ����ŵ�����CO32-���缫����ʽΪ��
HOCH2CH2OH-10e-+14OH-=2CO32-+10H2O���ʴ�Ϊ������HOCH2CH2OH-10e-+14OH-=2CO32-+10H2O��
���������⿼���˸�˹���ɵ�Ӧ�á�ƽ����ƶ���ȼ�ϵ�ص缫��Ӧ����д�����ݣ��ۺ��Խ�ǿ���Ѷ����У�

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A�����ȷ�Ӧ�ڳ����¶����Է����� |
| B��Fe3++3H2O?Fe��OH��3+3H+�����ȷ�Ӧ |
| C����ֵ��С��ϵΪ��S��������S��ˮ����S��ˮ������ |
| D����C��s��+H2O��g��?CO��g��+H2��g�������Щ̿�������ѧ��Ӧ���� |
������Ԫ��X��Y��Z��Ԫ�����ڱ��е����λ����ʾ������Xԭ�������������Ǵ�����������3��������˵��������ǣ�������
| X | |
| Y | Z |
| A��Ԫ��X��Ԫ��Y����������ϼ���ͬ |
| B����̬�⻯������ȶ��ԣ�H2Y��H2X |
| C��Y2Z2�ǹ��ۻ����� |
| D�����ԣ�HZO4��H2YO4 |
���ֶ�����Ԫ�������ڱ��е�λ����ͼ������ֻ��MΪ����Ԫ�أ�����˵������ȷ���ǣ�������

| A��ԭ�Ӱ뾶Z��M |
| B��Zλ��Ԫ�����ڱ��е�2���ڡ��ڢ�A�� |
| C��X�������̬�⻯������ȶ��Ա�Z��ǿ |
| D��Y������������Ӧˮ��������Ա�X��ǿ |
̫���ܵ����Ϊ��������Ϥ��̫���ܵ�ذ�İ뵼������ǣ�������
| A���������� | B���� |
| C���ѺϽ� | D�����Ͻ� |
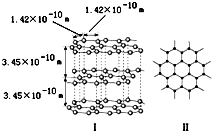 ��1��ʯī�Dz�״�ṹ������ͼ��ʾ��ÿһ���ڵ�̼ԭ�Ӽ�ͨ��sp2�ӻ��γ�
��1��ʯī�Dz�״�ṹ������ͼ��ʾ��ÿһ���ڵ�̼ԭ�Ӽ�ͨ��sp2�ӻ��γ� �����ᣨH3PO2����һ�־�ϸ������Ʒ����һԪ��ǿ�ᣬ���н�ǿ��ԭ�ԣ�
�����ᣨH3PO2����һ�־�ϸ������Ʒ����һԪ��ǿ�ᣬ���н�ǿ��ԭ�ԣ�
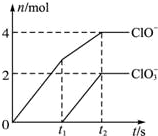 ��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������������ֺ���Ԫ�ص����ӣ������������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ����֪������ClO3-�ķ�ӦΪ��6Ca��OH��2+6Cl2�T5CaCl2+Ca��ClO3��2+6H2O��
��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������������ֺ���Ԫ�ص����ӣ������������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ����֪������ClO3-�ķ�ӦΪ��6Ca��OH��2+6Cl2�T5CaCl2+Ca��ClO3��2+6H2O��