��Ŀ����
�⣨Ԫ�ط���Mo��������ɫ�����۽������������ϼ�Ϊ+6��+5��+4�����������ڿ����к��ȶ�������600��ʱ�ܿ�����������������⣨MoO3����
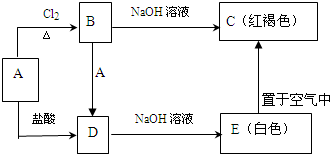
���⾫����Ҫ�ɷ�ΪMoS2�������������Ҫԭ�ϣ���ȡ���̰����������գ��������⡢��ۺ����������ȡ����Ҫ���裬����������ͼ��

��1�����⾫����600���½����������� ת��ΪMoO3��ͬʱ����SO2���壮��Ҫ��Ӧ�Ļ�ѧ����ʽΪ�� ����ұ�����Դ�������Ⱦ��Ҫ������ ������Ϊ���ò������ø��������ô�ʩ�� ��
��2�������������ȡ
����ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ����Ӧ�����ӷ���ʽΪ�� ��
�÷�Ӧ�� �����ǻ��ǣ�������ԭ��Ӧ��
�����������[��NH4��2MoO4]��Һ���ȵ�55��65�棬�������������Һ��pHΪ2�����������������[��NH4��2O?mMoO3?nH2O]���壻Ϊ��ȥ�ơ�þ���Ƶ����ʣ�����������������ڰ�ˮ�γ�����泥�ʹ���ӷ�������������茶���[��NH4��2O?7MoO3?4H2O]����������茶�����ˮ�����յô���Ϊ99.95%���������⣨MoO3����
��3��������۵���������ҵ���ڹ�״��¯����������������ԭ��������õ���ۣ�
����450��650���£�MoO3+3H2�TMoO2+3H2O
����900��950���£�MoO2+2H2�TMo+2H2O
ijͬѧ����������ԭԭ������Ϊ������ �Ȼ�ԭ����ԭMoO3�õ���ۣ�
���⾫����Ҫ�ɷ�ΪMoS2�������������Ҫԭ�ϣ���ȡ���̰����������գ��������⡢��ۺ����������ȡ����Ҫ���裬����������ͼ��

��1�����⾫����600���½����������� ת��ΪMoO3��ͬʱ����SO2���壮��Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
��2�������������ȡ
����ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ����Ӧ�����ӷ���ʽΪ��
�÷�Ӧ��
�����������[��NH4��2MoO4]��Һ���ȵ�55��65�棬�������������Һ��pHΪ2�����������������[��NH4��2O?mMoO3?nH2O]���壻Ϊ��ȥ�ơ�þ���Ƶ����ʣ�����������������ڰ�ˮ�γ�����泥�ʹ���ӷ�������������茶���[��NH4��2O?7MoO3?4H2O]����������茶�����ˮ�����յô���Ϊ99.95%���������⣨MoO3����
��3��������۵���������ҵ���ڹ�״��¯����������������ԭ��������õ���ۣ�
����450��650���£�MoO3+3H2�TMoO2+3H2O
����900��950���£�MoO2+2H2�TMo+2H2O
ijͬѧ����������ԭԭ������Ϊ������
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������,Ԫ�ؼ��仯����
��������1��������Ϣ�����⾫����600���½����������գ�ת��ΪMoO3��ͬʱ����SO2��������д����ʽ�������������ж����ʣ����Խ��л��գ����ݶ��������Ӧ�����ش�
��2��������Ϣ������ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ����д����ʽ���л��ϼ۱仯�ķ�Ӧ��������ԭ��Ӧ��
��3�����л�ԭ�Ե����ʣ�������һ����̼�ȣ�
��2��������Ϣ������ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ����д����ʽ���л��ϼ۱仯�ķ�Ӧ��������ԭ��Ӧ��
��3�����л�ԭ�Ե����ʣ�������һ����̼�ȣ�
���
��1�����⾫����600���½����������գ�ת��ΪMoO3��ͬʱ����SO2����2MoS2+7O2=2MoO3+4SO2�������������ж����ʣ����Խ��л���������������������ͻ��ʣ��ʴ�Ϊ��2MoS2+7O2=2MoO3+4SO2��SO2������SO2������ �������SO2������������ͻ��ʣ���
��2��������Ϣ������ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ������MoO3+2NH3?H2O=2NH4++MoO42-+H2O��û�л��ϼ۱仯������������ԭ��Ӧ���ʴ�Ϊ��MoO3+2NH3?H2O=2NH4++MoO42-+H2O�����ǣ�
��3������������ԭ��������õ���ۣ����Եó����л�ԭ�Ե�COҲ���Ի�ԭ��������õ���ۣ��ʴ�Ϊ��CO��
��2��������Ϣ������ɰ����Ҫ�ɷ֣�MoO3���ð�ˮ�������백ˮ��Ӧ���������[��NH4��2MoO4]��Һ������MoO3+2NH3?H2O=2NH4++MoO42-+H2O��û�л��ϼ۱仯������������ԭ��Ӧ���ʴ�Ϊ��MoO3+2NH3?H2O=2NH4++MoO42-+H2O�����ǣ�
��3������������ԭ��������õ���ۣ����Եó����л�ԭ�Ե�COҲ���Ի�ԭ��������õ���ۣ��ʴ�Ϊ��CO��
������������һ���������ʵķ����Ʊ�������ʵ������⣬����ѧ�������ͽ��������������Ѷȴ�

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
�Ƚ�MnO2��CuO��H2O2�ֽⷴӦ�Ĵ�������С��ʵ���У���������������������0.1g��3%��H2O2��Һ��ȡ5mL����ѡ���ʵ��װ���ǣ�������
A��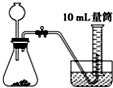 |
B��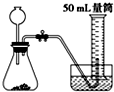 |
C��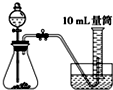 |
D�� |
��������Լ��ķ����У��д����һ�����Σ�յ��ǣ�������
| A��������ʢ��CS2�ij����Լ�ƿ�� |
| B�������ƽ���ʢ��ú�͵��Լ�ƿ�� |
| C��Ũ����������ɫ�Լ�ƿ�� |
| D����ˮ���ڴ����������Լ�ƿ�� |
�����и�����Һ�У�����һ���ܴ���������ǣ�������
| A������0.1 mol?L-1H+����Һ�У�NO3-��Na+��Cl-��Fe2+ |
| B����ɫ������Һ�У�K+��Mg2+��Cl-��SO42- |
| C��ǿ������Һ�У�Na+��SO42-��HCO3-��K+ |
| D����������Ӧ����H2����Һ�У�Na+��Al3+��Cl-��SO42- |
 ���ܱ������н������¿��淴Ӧ��A��g��+B��g��?C��g��+2D���������ڲ�ͬ��������C�İٷֺ����ı仯�������ͼ����÷�Ӧ������ȷ���ǣ�������
���ܱ������н������¿��淴Ӧ��A��g��+B��g��?C��g��+2D���������ڲ�ͬ��������C�İٷֺ����ı仯�������ͼ����÷�Ӧ������ȷ���ǣ�������| A������Ӧ���ȣ�D�ǹ��� |
| B������Ӧ���ȣ�D������ |
| C������Ӧ���ȣ�D������ |
| D������Ӧ���ȣ�D�ǹ�������� |