��Ŀ����
17�������������ʣ��ٸɱ� ��NaHCO3���� �۰�ˮ �ܴ������FeCl3��Һ ��ͭ ��Fe��OH��3���� �����ǣ�
��1���������ڵ���ʵ��Ǣڢܣ�
��2��д��NaOH��aq�� ��Al��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��3��������һ�ֳ����ķ�ɢϵ���ش��������⣮
����Fe��OH��3�����м���Na2SO4������Һ������SO42-���ӣ������ӷ��ţ������ã�ʹ�����γ��˳�����������̽�������ľ۳���
�����ֽ������Һ���õķ������������ЧӦ��
���� ��1����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
��2�������������Ʒ�Ӧ����ƫ�����ƺ�������
��3��������е�����ˮ����μ���FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȣ����Ƶ�Fe��OH��3���壻
�ڽ���������ʷ����۳���
�۽�����ж����ЧӦ��
��� �⣺��1���ڢ���ˮ��Һ�������״̬���ܵ�����������Ӷ�ʹ��ˮ��Һ������״̬���磬�������ڵ���ʣ��ʴ�Ϊ���ڢܣ�
��2�������������Ʒ�Ӧ����ƫ�����ƺ����������ӷ�ӦΪ2Al+2OH-+2H2O�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��3������Fe��OH��3�����м���Na2SO4������Һ����������������к����������������ĵ�ɣ����½��巢���˾۳����ʴ�Ϊ��SO42-��
�ڽ�����ж����ЧӦ���ݴ˿������ֽ������Һ������Ķ����ЧӦ�ǽ������ӶԹ��ߵ�ɢ�������γɵģ��ʴ�Ϊ�������ЧӦ��
���� ���⿼���˵���ʡ��ǵ���ʵĸ�����ӷ�Ӧ����ʽ����д��������Ʊ������ʵȣ��Ƚϻ��������ضԻ���֪ʶ�Ĺ��̣�ע��Ի���֪ʶ���������գ���Ŀ�ѶȲ���ע�����ս�����Ʊ���������������ʼ���������ȷ�������ǵ���ʵ�����

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д���1����ҵ�����ø�����FeO Cr2O3��ұ�����Ĺ����������£�
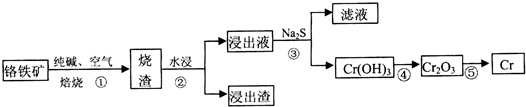 a����������Ϊ���ȱ������ʺ����ԭ�ϵ������ʣ��ɲ�ȡ�Ĵ�ʩ�ǽ���������飨��һ�����ɣ���
a����������Ϊ���ȱ������ʺ����ԭ�ϵ������ʣ��ɲ�ȡ�Ĵ�ʩ�ǽ���������飨��һ�����ɣ���b������Һ�к��е���Ҫ�ɷ�ΪNa2CrO4������Һ�м��ữ���Ȼ�����Һ�а�ɫ�������ɣ�������۷�����Ӧ�����ӷ���ʽCrO42-+Ba2+=BaCrO4����
��2�����Ļ������ж����ŷ�ǰҪ����������ͨ���õ�ⷨ��
����Cr2O72-�ķ�ˮ��������ڣ�����һ�����Ȼ��ƣ��������缫�ṩFe2+��Cr2O72-��Fe2+��������Fe3+����������ԭ����Cr3+�����Cr3+��Fe3+��Cr��OH��3��Fe��OH��3����ʽ��ȥ���������۵�������������ӦʽΪ2H++2e-=H2����Cr2O72-����Fe2+�����ӷ���ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
��3��CrO3��һ�ֳ����ĸ�������������ȶ��Խϲ����ʱ�ֽ⣮ȡ100gCrO3���ȣ�ʣ�������������¶ȵı仯���±���ʾ��
| �¶�/K | 480 | 505 | 615 | 730 |
| ����/g | 94.67 | 92.00 | x | 76.00 |
| ��ѧʽ | Cr3O8 | y | CrO2 | Cr2O3 |
��K+��Cl-��NO3-��CH3COO-����K+��Fe2+��I-��SO42-
��Ba2+��Cl-��NO3-��Na+����Na+��Ca2+��Cl-��HCO3-
��K+��SO42-��Cl-��NO3-��
| A�� | �� | B�� | �٢ۢ� | C�� | �ڢ� | D�� | �ڢۢ� |
| A�� | Na2CO3��s�� | B�� | ˮ | C�� | �������Һ | D�� | ���� |
| A�� | �������뻹ԭ�������ʵ���֮��Ϊ1��6 | |
| B�� | ���������뻹ԭ���������֮��Ϊ1��1 | |
| C�� | ����3molˮʱ��1mol KIO3������ | |
| D�� | 1mol KIO3�μӷ�Ӧʱ��5mol����ת�� |
| A�� | HF��HCl��HBr��HI���۵�е��������� | |
| B�� | HF��HCl��HBr��HIˮ��Һ�������������� | |
| C�� | �Ҵ�������ˮ������Ϊ�Ҵ�������ˮ����֮��ֻ���ڷ��»��� | |
| D�� | �ȵĸ��ֺ������������ǿ��������ΪHClO��HClO2��HClO3��HClO4 |
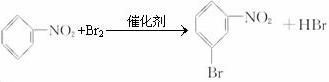 ��
�� ��
��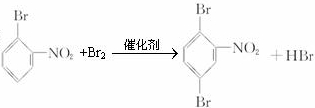 ��
��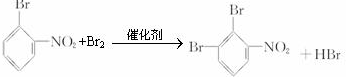 ��
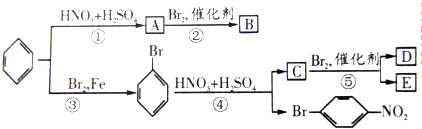
��
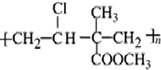 ���䵥��Ϊ��CH2=CHCl��CH2=C��CH3��COOCH3��
���䵥��Ϊ��CH2=CHCl��CH2=C��CH3��COOCH3��