��Ŀ����
2���������棨CeO2����һ����Ҫ��ϡ���������ҵ���ԷϾ���ʾ����������CeO2��Fe2O3��FeO��SiO2�ȣ�Ϊԭ�ϣ�����ȡCe��OH��4��������立�[Fe2��SO4��3•��NH4��2SO4•24H2O]���乤��������ͼ��
��֪��
�����������£�����ˮ��Һ��Ce3+��Ce4+������Ҫ��Ҳ����ʽ��
��CeO2������ϡ���ᣬҲ������NaOH��Һ�����н�ǿ�����ԣ�
�ش��������⣺
��1����Ӧ�ٵ����ӷ���ʽ��SiO2+2OH-�TSiO32-+H2O
��2��ϴ�O����B��Ŀ����Ϊ�˳�ȥFe3+��Fe2+���������ӷ��ţ�
��3����Ӧ�۵����ӷ���ʽ��2CeO2+H2O2+3H2SO4�TCe2��SO4��3+O2��+4H2O��
��4����ȡ�Ƿ���ϡ��Ԫ�صij��÷�������֪�л���HT�ܽ�Ce3+��ˮ��Һ����ȡ�������ù��̿ɱ�ʾΪ��2Ce3+��ˮ�㣩+6HT���л��㣩?2CeT3���л��㣩+6H+��ˮ�㣩����ƽ��ǶȽ��ͣ���CeT3���л��㣩����H2SO4��ýϴ��ĺ�Ce3+��ˮ��Һ��ԭ���Ǽ���H2SO4����Һ��������Ũ������ƽ�����γ�Ce3+ˮ��Һ�ķ����ƶ���
��5������������[Fe2��SO4��3•��NH4��2SO424H2O]���㷺����ˮ�ľ����������侻ˮԭ�������ӷ���ʽ������Fe3++3H2O?Fe��OH��3�����壩+3H+��
��6��pH��ͬ������������Һ�У�NH${\;}_{4}^{+}$��Ũ�ȴ�С��ϵΪb=d��a��c��
a��Fe2��SO4��3•��NH4��2SO4•24H2O
b����NH4��2SO4
c��NH4HSO4
d��NH4Cl
��7��ȡ���������еõ���Ce��OH��4��Ʒ0.832g���������ܽ����0.1000mol•L-1 FeSO4����Һ�ζ����յ�ʱ������38.00 mL����Һ���ò�Ʒ��Ce��OH��4����������Ϊ95.0%�������������λ��Ч���֣���������FeSO4����Һ�ڿ�����¶��һ��ʱ����ٵζ������ø�Ce��OH��4��Ʒ����������ƫ���ƫ��ƫС������Ӱ�족����
���� �ϲ�����ĩ����CeO2��Fe2O3��FeO��SiO2�ȣ�������������Һ�������������������Ʒ�Ӧ���ɹ����ƣ����ˣ��õ�����A�ijɷ���Fe2O3��FeO��CeO2����ҺAΪ��������Һ������A��Fe2O3��FeO��CeO2����ϡ�������˵���ҺB��������������������Һ������B�ijɷ���CeO2������B�е�CeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��O2�����л���HT��Ce3+��ˮ��Һ����ȡ������Ce3+�м����������Ce��OH��4������������ҺB�õ���ҺC���ټ�����淋õ�Fe2��SO4��3•��NH4��2SO4•24H2O��
��1�����ݷϲ�����ĩ�ijɷ֣���SiO2��Fe2O3��FeO��CeO2�ȣ�����֪�����������Ʒ�Ӧ��ֻ�ж������裬���ɹ����ƣ�
��2��������Һ�е����ӷ�����
��3���ڢ۲���ӦCeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��CeԪ����+4�۱�Ϊ+3�ۣ�����ԭ����H2O2Ӧ����������O2��Ȼ����ƽ�ó��ڢ۲���Ӧ�Ļ�ѧ����ʽ��
��4������Ӱ�컯ѧƽ���ƶ������ط�����
��5������������Һ��ˮ�����������������壬������ˮ�е�����������
��6������������笠����ӵ�ˮ�⣬������������Ҳ������笠����ӵ�ˮ�⣬ˮ��̶�Խ��笠�����Ũ��ԽС��
��7�����ݵ����غ㽨����ϵʽ��Ce��OH��4��FeSO4��Ȼ����м������Ce��OH��4������������������������FeSO4�ڿ������ױ���������������FeSO4��Ũ�ȼ�С��
��� �⣺�ϲ�����ĩ����CeO2��Fe2O3��FeO��SiO2�ȣ�������������Һ�������������������Ʒ�Ӧ���ɹ����ƣ����ˣ��õ�����A�ijɷ���Fe2O3��FeO��CeO2����ҺAΪ��������Һ������A��Fe2O3��FeO��CeO2����ϡ�������˵���ҺB��������������������Һ������B�ijɷ���CeO2������B�е�CeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��O2�����л���HT��Ce3+��ˮ��Һ����ȡ������Ce3+�м����������Ce��OH��4������������ҺB�õ���ҺC���ټ�����淋õ�Fe2��SO4��3•��NH4��2SO4•24H2O��
��1���ϲ�����ĩ�ijɷ֣���SiO2��Fe2O3��FeO��CeO2�ȣ��������������Ʒ�Ӧ��ֻ�ж������裬��Ӧ���ɹ����ƣ��䷴Ӧ�����ӷ���ʽΪ��SiO2+2OH-�TSiO32-+H2O��
�ʴ�Ϊ��SiO2+2OH-�TSiO32-+H2O��
��2���ڢڲ���ӦFe2O3��ϡ������������Fe2��SO4��3��FeSO4��ϴ������B��Ŀ����Ȼ��Ϊ�˳�ȥFe3+��Fe2+���ʴ�Ϊ��Fe3+��Fe2+��
��3���ڢ۲���ӦCeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��CeԪ����+4�۱�Ϊ+3�ۣ�����ԭ����H2O2Ӧ����������O2��Ȼ����ƽ�ó��ڢ۲���Ӧ�Ļ�ѧ����ʽ 2CeO2+H2O2+3H2SO4�T2Ce2��SO4��3+O2��+4H2O��
�ʴ�Ϊ��2CeO2+H2O2+3H2SO4�TCe2��SO4��3+O2��+4H2O��
��4���л���HT�ܽ�Ce3+��ˮ��Һ����ȡ�������ù��̿ɱ�ʾΪ��2Ce3+��ˮ�㣩+6HT���л��㣩?2CeT3���л��㣩+6H+��ˮ�㣩����CeT3���л��㣩����H2SO4����Һ��������Ũ������ƽ�����γ�Ce3+ˮ��Һ�ķ����ƶ���
�ʴ�Ϊ������H2SO4��Һ��������Ũ������ƽ�����γ�Ce3+ˮ��Һ�ķ����ƶ���
��5��Fe2��SO4��3•��NH4��2SO4•24H2O����Һ�е���������ӣ�����������Һ��ˮ�����������������壬Fe3++3H2O?Fe��OH��3�����壩+3H+��������������������ˮ�е������������Ӷ���ˮ���ã�
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3�����壩+3H+��
��6��a��Fe2��SO4��3•��NH4��2SO4•24H2O��Һ��NH4+��������ˮ�������ԣ�
b����NH4��2SO4��Һ��NH4+ˮ�������ԣ�
c��NH4HSO4��Һ���������������ȫ����������ӣ�
d��NH4Cl��Һ��NH4+ˮ�������ԣ�
����pH��ͬʱNH4+Ũ�ȴ�С��ϵΪb=d��a��c��
�ʴ�Ϊ��b=d��a��c��
��7��Ce��OH��4 ��FeSO4
0.00380mol 0.1000mol/L-1��0.0380L
����m��Ce��OH��4��=0.00380mol��208g/mol=0.7904g����Ʒ��Ce��OH��4����������Ϊ$\frac{0.7904}{0.832}$��100%=95.0%��
FeSO4�ڿ������ױ���������������FeSO4��Ũ�ȼ�С���ζ�ʱ���ĵı���Һ�����ƫ�����Լ���õ���Ce��OH��4������ƫ������������ƫ��
�ʴ�Ϊ��95.0%��ƫ��
���� �����Թ�������Ϊ֪ʶ������������ѧ�������ʷ����ᴿ�����ķ��������ͶԻ���֪ʶ���ۺ�Ӧ�ÿ��飬��Ŀ�Ѷ��еȣ�ע����չ��������Լ�Ԫ�ػ���������ʣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�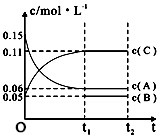
| A�� | ��a=3����b=1��c=2 | |
| B�� | t1minʱ���÷�Ӧ�ﵽ�������µķ�Ӧ�� | |
| C�� | ��O��t1min�ڣ���C��ʾ�Ļ�ѧ��Ӧ����Ϊ0.06mol•L-1 | |
| D�� | B����ʼŨ�ȵ���0.08mol•L-1 |
| A�� | Fe2++2NH3•H2O�TFe��OH��2��+2NH4+ | |
| B�� | Fe2++NH3•H2O+HCO3-�TFeCO3��+NH4++H20 | |
| C�� | Fe2++2HCO3-�TFe��OH��2��+2CO2�� | |
| D�� | 2Fe2++HCO3-+3NH3•H2O�TFe2��OH��2CO3��+3NH4++H2O |
| Ԫ�ش��� | A | B | C | D | E | F | G | H |
| ԭ�Ӱ뾶/pm | 37 | 160 | 70 | 66 | 186 | 143 | 104 | 99 |
| ����ϼ� | +1 | +2 | +5 | +1 | +3 | +6 | +7 | |
| ��ͻ��ϼ� | -3 | -2 | -2 | -1 |
��1��G��Ԫ�����ڱ��е�λ���ǵ�������VIA�壨�����ں��壩
��2����������Ԫ���У�����������ˮ����������ǿ����HClO4���ѧʽ������̬�⻯��ˮ��ҺpH��7����NH3���ѧʽ����
��3��B��D��E��F����Ԫ�ص����ӣ������Ӱ뾶������O2-�������ӷ��ţ���
��4��A2D�ĵ���ʽ��
 ��B��H����Ԫ���γɻ�����ĵ���ʽ��
��B��H����Ԫ���γɻ�����ĵ���ʽ�� ��
����5��A��C��H����Ԫ����ɵĻ�����Ļ�ѧʽ��NH4Cl���û������������ӻ��������ӡ����ۡ�����
��6��E��F����Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
 ����X��������ͺ˴Ź���������������ԭ�ӵĻ�ѧ����ֻ��һ�֣����ݷ���������˵���в���ȷ���ǣ�������
����X��������ͺ˴Ź���������������ԭ�ӵĻ�ѧ����ֻ��һ�֣����ݷ���������˵���в���ȷ���ǣ�������| A�� | X�ķ���ʽΪC5H4 | |
| B�� | X��̼ԭ�ӵĻ�ѧ������2�� | |
| C�� | 1 mol X��һ�������¿���2 mol����������Ӧ | |
| D�� | X����ʹ���Ը��������Һ��ɫ |
 ��ش��������⣮
��ش��������⣮��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�
��100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��KW��25�棩��KW��100�棩�����������������=������
��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����
��2������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݣ�
| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
��25��ʱ����Ũ�ȵ�CH3COOH��Һ��NaOH��Һ�������ϣ���c��Na+����c��CH3COO-�������������������=������
| Ԫ�ر�� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/nm | 0.037 | 0.074 | 0.082 | 0.099 | 0.102 | 0.143 | 0.152 |
| ����ϼۻ���ͻ��ϼ� | +1 | -2 | +3 | -1 | -2 | +3 | +1 |
| A�� | �ߢٿ��γ����ӻ����� | |
| B�� | Ԫ�آڢ��γɵĻ������������ | |
| C�� | Ԫ�آ��⻯��ķе�С��Ԫ�آ��⻯��ķе� | |
| D�� | Ԫ�آ���̬�⻯����ȶ��Դ���Ԫ�آ���̬�⻯����ȶ��� |
