��Ŀ����
9��������ѧ֪ʶ��������пհ״����ݣ���1��ȡ23g�ơ�24gþ��27g���ֱ�������ϡ���ᷴӦ������ͬ�����£��������������֮����1��2��3��
��2������������Na2CO3��NaHCO3���ְ�ɫ�����ʵ�������ABC��ѡ��������ĸ����
A���ֱ���������ֹ������ʣ��������ɵ�����ͨ������ʯ��ˮ��
B���ֱ������������ʵ���Һ�У�����CaCl2��Һ
C���ֱ�����������Һ�У�����ͬŨ�ȵ�ϡ����
D���ֱ������������ʵ���Һ�У��������������ʯ��ˮ
��3��һ�������մ��С�մ����ֱ���������ϡ���ᷴӦ������������������ڱ���¾�Ϊ33.6L�����������HCl�����ʵ���֮��Ϊ2��1����Ҫ���ߵĹ�������֮��Ϊ53��42��
���� ��1���漰���Ļ�ѧ����ʽ�Т�2Na+2HCl=2NaCl+H2������Mg+2HCl=MgCl2+H2������2Al+6HCl=2AlCl3+3H2��������Ŀ��������Ϸ���ʽ���м��㣻
��2��Na2CO3��NaHCO3���ʲ�ͬ����NaHCO3���ȷֽ⣬��̼���������ˮ����̼���������ˮ����NaHCO3�����ᷴӦ���ң��ܶ��ܳ���ʯ��ˮ��Ӧ����̼��Ƴ������ݴ˽��з�����
��3����Na2CO3+2HCl=2NaCl+H2O+CO2����NaHCO3+HCl=NaCl+H2O+CO2�����в��������������HCl�����ʵ���֮�ȣ����ߵĹ�������֮�ȣ�
��� �⣺��1��2Na+2HCl=2NaCl+H2��
46 22.4
23 11.2
��V��H2��=11.2L��
Mg+2HCl=MgCl2+H2��
24 22.4
24 22.4
��V��H2��=22.4L��
2Al+6HCl=2AlCl3+3H2��
54 67.2
27 33.6
��V��H2��=33.6L���ʲ������������֮��11.2��22.4��33.6=1��2��3��
�ʴ�Ϊ��1��2��3��
��2��A��Na2CO3���Ȳ��ֽ⣬��NaHCO3���ȷֽ⣬����������ʹ�����ʯ��ˮ����ǣ����Լ��𣬹�A��ȷ��
B��Na2CO3��CaCl2��Һ���ò���CaCO3��������NaHCO3����CaCl2��Һ��Ӧ���������������Լ��𣬹�B��ȷ��
C����Na2CO3����μ�������ʱ������������������������϶�ʱ���ɲ������壬����NaHCO3�м���ͬŨ�����ᣬ��Ѹ�ٲ������壬����ͨ����������Ŀ������Լ��𣬹�C��ȷ��
D��Na2CO3��NaHCO3���������ʯ��ˮ��Ӧ����������������ͬ�������𣬹�D����
�ʴ�Ϊ��ABC��
��3��n��CO2��=$\frac{33.6L}{22.4L/mol}$=1.5mol��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 2 1
159 3 1.5
��n��HCl��=3mol��m��Na2CO3��=159g��
NaHCO3+HCl=NaCl+H2O+CO2����
84 1 1
126 1.5 1.5
��n��HCl��=1.5mol��m��NaHCO3��=126g��
��HCl�����ʵ���֮��Ϊ3��1.5=2��1�����ߵĹ�������֮��Ϊ159��126=53��42��
�ʴ�Ϊ��2��1��53��42��
���� ����Ϊ�ۺ�����Ŀ�������ǣ�2���������ʵļ�����Ŀ�ѶȲ���ע��̼���ƺ�̼�����Ƶ����ʵ���ͬ��ѧϰ��ע����ػ���֪ʶ�Ļ��ۣ�

| A�� | ���ȿɼӿ췴Ӧ���� | B�� | ����ϡ�����Ũ�ȿɼӿ췴Ӧ���� | ||
| C�� | ����п���ɼӿ췴Ӧ���� | D�� | ���п�Ĵ��ȿɼӿ췴Ӧ���� |
| A�� | 60g�������еĹ��ۼ���ĿΪ10NA | |
| B�� | ���³�ѹ�£�4.4gCO2��N2O��������к��е�ԭ����Ϊ0.3NA | |
| C�� | �ڹ���������ˮ�ķ�Ӧ�У�ÿ����0.1mol������ת�Ƶ��ӵ���ĿΪ0.4NA | |
| D�� | 80ml10mol/L����������MnO2���ȷ�Ӧ������Cl2�ķ�����Ϊ0.2NA |
| A�� | ���ѣ��ף����ѣ��ң����������ѹǿ���ף��� | B�� | ���ѣ��ף����ѣ��ң�������������ף��� | ||
| C�� | ���ѣ��ף����ѣ��ң���������Ħ��������ף��� | D�� | ���ѣ��ף����ѣ��ң���������������ף��� |
| A�� | ȼ�ŵľƾ��ƴ�ʧ��������ˮ���� | |
| B�� | ��������Ũ��Һմ��Ƥ���ϣ������ô���ˮ��ϴ����Ϳ��������ϡ������Һ | |
| C�� | ��������ԭ����ͭʱ���ȼ�����ͨ�������������˷����� | |
| D�� | ����ϡ����ʱ����������Ͳ�м���һ������ˮ�����ڽ����»�������Ũ���� |
| A�� | 80g CuO��Cu2S�Ļ�����У�����ͭԭ����ΪNA | |
| B�� | 1mol NaBH4������ˮ��Ӧ��NaBH4+H2O��NaBO2+H2����δ��ƽ��ʱת�Ƶĵ�����Ϊ4NA | |
| C�� | �����£�2L 0.1 mol•L-1FeCl3 ��Һ�� 1L 0.2 mol•L-1FeCl3 ��Һ���� Fe3+��Ŀ��ͬ | |
| D�� | 100g��������Ϊ46%���Ҵ���Һ�к���NA��-OH |
 ��
��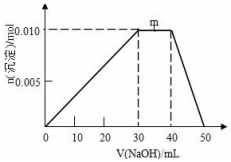 ��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£�
��ʢ��10mL1mol•L-1 NH4Al��SO4��2��Һ���ձ��еμ�1mol•L-1NaOH��Һ���������ʵ�����NaOH��Һ����仯ʾ��ͼ���£�