��Ŀ����
 ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������
ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�������������1����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ
��2����Һ©������ƿ�����齺�����ӵ�Ŀ����
��3��Ϊ�˵õ��Ƚ�ƽ�ȵ���Ȳ������ʵ����ͨ����
��4��ʵ���в��������Ͳ��ˮ�����ΪVL����ʯ������ΪWg�����ʯ��̼���Ƶ�����������
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ�������
��������1����ʯ��ˮ��Ӧ�ķ���ʽΪCaC2+2H2O��Ca��OH��2+C2H2����װ��BC����ˮ���������ⶨ������Ȳ�������
��2��������ƿ�ڵ�����ѹǿ��Һ��˳�����·����жϣ�
��3��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ������
��4�����ݷ�Ӧ������ϵCaC2-----C2H2������õ���Ȳ�������������ʯ��CaC2������������
��2��������ƿ�ڵ�����ѹǿ��Һ��˳�����·����жϣ�
��3��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ������
��4�����ݷ�Ӧ������ϵCaC2-----C2H2������õ���Ȳ�������������ʯ��CaC2������������
���
�⣺��1����ʯ��ˮ��Ӧ�ķ���ʽΪCaC2+2H2O��Ca��OH��2+C2H2����װ��B������ˮ�Ա���ƿ�����ɵ�C2H2�������Bʱ�ų������������ˮ������ͲC���ⶨ������Ȳ������
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+C2H2������ˮ������
��2����Ӧ��������ƿ�ڵ�����ѹǿ��ʹ��Һ©���ڵ�Һ�����ڵ���ƿ�У��������齺�ܽ���Һ©������ƿ���ӳ�һ������������Һ��˳�����£�
�ʴ�Ϊ��ƽ��ѹǿ������Һ��˳�����£�
��3��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ�������ʴ�Ϊ������ʳ��ˮ��
��4�������VmL������ҪCaC2������Ϊmg����
CaC2-----C2H2��
64g 22.4L
m VL
���m=2.9V�����ʯ��CaC2����������Ϊ
��100%��
�ʴ�Ϊ��
��100%��
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+C2H2������ˮ������
��2����Ӧ��������ƿ�ڵ�����ѹǿ��ʹ��Һ©���ڵ�Һ�����ڵ���ƿ�У��������齺�ܽ���Һ©������ƿ���ӳ�һ������������Һ��˳�����£�
�ʴ�Ϊ��ƽ��ѹǿ������Һ��˳�����£�
��3��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ�������ʴ�Ϊ������ʳ��ˮ��
��4�������VmL������ҪCaC2������Ϊmg����
CaC2-----C2H2��
64g 22.4L
m VL
���m=2.9V�����ʯ��CaC2����������Ϊ
| 2.9V |
| W |
�ʴ�Ϊ��
| 2.9V |
| W |
���������⿼�����������ʵ�̽��ʵ������жϣ�Ϊ��Ƶ���㣬������ѧ����ʵ�����ݵķ����ͼ��㣬��Ȳ���ʺ��Ʊ�������ʵ�����������ʵ����Ʒ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
����˵����ȷ���ǣ������������ǻ�����BaSO4��һ��������ˮ�ĵ���ʢ����ᡢ���С�մ�ֱ������ᡢ���������ԭ��Ӧ���������ӷ�Ӧ��������ָpHС��7.0�Ľ�ˮ�����ЧӦ����������Һ�ͽ��壮������ȷ���ǣ�������
| A���٢ڢ� | B���٢ܢ� |
| C���ۢ� | D���ڢ� |
������ͼ��ʾʵ��װ�õ�����������ǣ�������


| A����ˮ�����ᣬҺ������������Ҳ��½���˵��װ�ò�©�� |
| B������֪�μӷ�Ӧ��Zn�����������װ�ÿ������ⶨ����Ħ����� |
| C������֪�μӷ�Ӧ��Zn�����������װ�ÿ������ⶨп�����ԭ������ |
| D����Ӧֹͣ���������� |
ʵ��������240mL 0.1mol?L-1������ͭ��Һ����ѡȡ250mL����ƿ�������ƣ����²��������Ƴɹ����ǣ�������
| A����ȡ3.84 g CuSO4��ĩ������250 mLˮ |
| B����ȡ6.25 g CuSO4?5H2O���壬��ˮ���250 mL��Һ |
| C����ȡ4.0 g CuSO4��ĩ������250 mLˮ |
| D����ȡ4.0 g CuSO4?5H2O���壬��ˮ���250 mL��Һ |
����ʵ��������������ˮ�е�ijЩ�ɷ֣����������ʣ�û�й�ϵ���ǣ�������
| A����NaHCO3�������������ˮ�У�����ɫ���ݣ�H+�� |
| B��ʹ��ɫ������ɫ��HCl�� |
| C����FeCl2��Һ�еμ���ˮ���ٵμ�KSCN��Һ�����ֳʺ�ɫ��Cl2�� |
| D���μ�AgNO3��Һ���ɰ�ɫ������Cl-�� |
ij��ɫ����ǿ������Һ�У��ܴ��������һ�������ǣ�������
| A��K+��Na+��AlO2-��SO42- |
| B��Mg2+��Al3+��NO3-��SO42- |
| C��Na+��K+��SO42-��MnO4- |
| D��NH4+��Na+��HCO3-��NO3- |

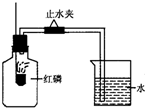 ��֪��������Ҫ�ɷ��ǵ�����������ij����С����Ʋⶨ����������������ʵ�飬ʵ��װ����ͼ��ʾ��
��֪��������Ҫ�ɷ��ǵ�����������ij����С����Ʋⶨ����������������ʵ�飬ʵ��װ����ͼ��ʾ��