��Ŀ����
13����Ϊ���ڱ���һ���֣����е���ĸ������Ӧ��Ԫ�أ��������ЩԪ�ػش��������⣮
��ش��������⣺
��1��Ԫ��I��Ԫ�ط���Cr����֪M2+����3d�������6�����ӣ����Ƴ�MԪ��λ�����ڱ���������VIB�壮
��2������Ԫ�ص�һ��������С����K����Ԫ�ط��ţ���ͬ�����縺��������F����ѧ�������ȶ�����Ar��
��3������Ԫ�ش���d������Ti��Cr����Ԫ�ط��ţ���
��4����ٳ�F�Ľ����Ա�Cǿ��ʵ����ʵ����ͬ�����£���ˮ��Ӧ�ر��ƾ��ң��������صļ��Ա���������ǿ��������ԭ�ӽṹ���۽�����ԭ�ص�ԭ�Ӱ뾶���ƴ�ԭ�Ӻ˶��������ӵ��������������Ƚ�����ʧ���ӣ�
���� ��Ԫ�������ڱ���λ�ÿ�֪��A��I�ֱ�ΪO��F��Na��Mg��Ar��K��Ca��Ti��Cr��
��1��Ԫ��IΪ����M2+����3d�������6�����ӣ�M�ļ۵���Ϊ3d64s2����ԭ������Ϊ18+2+6=26��ΪFeԪ�أ�
��2����������ǿ����һ��������С���ǽ�����Խǿ���縺��Խ��ϡ��������������ȶ���
��3���������d�����d����
��4��F�Ľ����Ա�Cǿ�������ý�����ˮ��Ӧ�ľ��ҳ̶�˵����
��� �⣺��Ԫ�������ڱ���λ�ÿ�֪��A��I�ֱ�ΪO��F��Na��Mg��Ar��K��Ca��Ti��Cr��
��1��Ԫ��IΪCr��M2+����3d�������6�����ӣ�M�ļ۵���Ϊ3d64s2����ԭ������Ϊ18+2+6=26��ΪFeԪ�أ�λ�����ڱ���������VIB�壬
�ʴ�Ϊ��Cr���ģ�VIB��
��2��K�Ľ�������ǿ����һ��������С��F�ķǽ�����Խǿ���縺��Խ��ϡ��������������ȶ�����Ar�Ļ�ѧ�������ȶ���
�ʴ�Ϊ��K��F��Ar��
��3���������d����λ��d������֪Ԫ�ش���d������Ti��Cr���ʴ�Ϊ��Ti��Cr��
��4��F�Ľ����Ա�Cǿ����ͬ�����£���ˮ��Ӧ�ر��ƾ��ң��������صļ��Ա���������ǿ������ԭ�ӽṹ���۽�����ԭ��Ϊ�ص�ԭ�Ӱ뾶���ƴ�ԭ�Ӻ˶��������ӵ��������������Ƚ�����ʧ���ӣ�
�ʴ�Ϊ����ͬ�����£���ˮ��Ӧ�ر��ƾ��ң��������صļ��Ա���������ǿ�����ص�ԭ�Ӱ뾶���ƴ�ԭ�Ӻ˶��������ӵ��������������Ƚ�����ʧ���ӣ�
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʼ��ṹ������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| ʵ�� | ҩƷ | ��ȡ���� | �������е�Һ�� |
| �� | Cu��ϡHNO3 | H2O | |
| �� | NaOH���塢Ũ��ˮ | NH3 | |
| �� | Na2SO3���塢ŨH2SO4 | SO2 | |
| �� | þ���Ͻ�NaOH��Һ�������� | H2 | H2O |
��2����ͬѧ��Ϊʵ��I��ͨ���ռ�������NO����������̽��ͭ��Ʒ�Ĵ��ȣ�����Ϊ�Ƿ���У������У�����С������С�����ԭ��ΪNO����װ���п�����Ӧ������NO2����ˮ��ʹ��õ�NO���������
��3��ʵ�����ƿ�в�����SO2����ͨ����ˮ��Һ�з�����Ӧ�����ӷ���ʽ��SO2+Br2+2H2O=4H++2Br-+SO42-��
��4��ʵ������������е�Һ�������c������ĸ��ţ���ͬ����
a��ŨNaOH��Һ b����ˮ c��ú�� d���Ȼ����Һ
��ʵ��ʣ���NH3�����մ�������ͼ2�����¸���β������װ���У��ʺ�������NH3�������ܷ�ֹ��������ACDF��
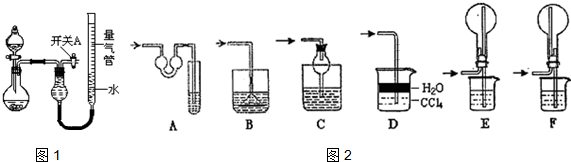
��5����ʵ��Ӧ�������ܶ�ζ���������ʱӦע�⣺�ٽ�ʵ��װ�ûָ������£���ʹ����������Һ����ƽ���������밼Һ����ʹ���ƽ��
��6��ʵ�������������ݣ�������������ѻ���ɱ�״�������Ե���Һ����������������Ӱ�죩
| ��� | þ���Ͻ���� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| �� | 1.0g | 10.0mL | 346.3mL |
| �� | 1.0g | 10.0mL | 335.0mL |
| �� | 1.0g | 10.0mL | 345.7mL |
| A�� | SO2��SiO2��CO��Ϊ���������� | B�� | ϡ���������ᡢ�Ȼ�����Һ��Ϊ���� | ||
| C�� | �ռ���ʯӢ��Ϊ����� | D�� | ��ˮ��ˮ��������ˮ��Ϊ����� |
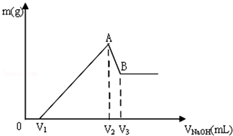 ��Mg��Al��ɵĻ���ﹲ0.1mol����100mL 3mol/LHCl��Һ�У��ٵμ�1mol/LNaOH ��Һ���ڵμ�NaOH��Һ�Ĺ����У�����������m��NaOH��Һ���V�ı仯��ͼ��ʾ��
��Mg��Al��ɵĻ���ﹲ0.1mol����100mL 3mol/LHCl��Һ�У��ٵμ�1mol/LNaOH ��Һ���ڵμ�NaOH��Һ�Ĺ����У�����������m��NaOH��Һ���V�ı仯��ͼ��ʾ��
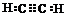 ��A�Ķ���ȡ������1�֣�
��A�Ķ���ȡ������1�֣�