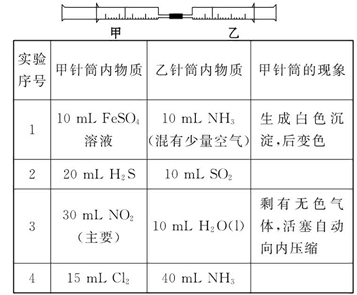
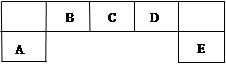��Ŀ����
A��B��C����ѧ��ѧ�������������ʣ�����֮����ת����ϵ���£����ַ�Ӧ������������ȥ����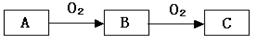
��1����A��һ�ֻ�ɫ���ʹ��壬��B��C�Ļ�ѧ����ʽΪ ��
��2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��C������Ϊ �����û�ѧ����ʽ��ʾ�������������̼����ķ�Ӧ ����C����¶���ڿ����У���������D��D�Ļ�ѧʽΪ ������D��NaHCO3�Ĺ�������10g���������������ٸı䣬ʣ���������Ϊ9.38 g��D����������Ϊ ��
��3����C�Ǻ���ɫ���壬A������һ����ʹʪ��ĺ�ɫʯ����ֽ���������塣��ͼ��ʵ������ȡA�����װ�ã�������ѧ֪ʶ���ش��������⣺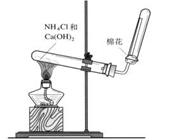
���ռ�A�ķ����� ����֤A�Ƿ��Ѿ��ռ����ķ����� ����дһ�֣���
��д��ʵ������ȡA�Ļ�ѧ����ʽ ��
������5��35g�Ȼ�鱗μӷ�Ӧ���������A�����ڱ�״���µ����Ϊ L��
����д��C��ˮ��Ӧ�Ļ�ѧ����ʽ ����Ӧ�ɵõ���X��X�� ��
���ʣ��ǿ����������������ͼ������X��Ũ��Һ��Cu��Ӧ��д����ƿ�з�����Ӧ�����ӷ���ʽ ��ʵ����Ϻ��Թ����ռ������������Ҫ�ɷ�Ϊ ��д��ѧʽ��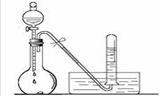
��1��2SO2��O2 2SO3
2SO3
��2���������� 2Na2O2��2CO2��2 Na2CO3��O2 Na2CO3 83.2%
��3���������ſ����� ��ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ�����
������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�����
��Ca(OH)2��2NH4Cl CaCl2��2NH3����2H2O ��2.24
CaCl2��2NH3����2H2O ��2.24
��3NO2��H2O��2 HNO3��NO ǿ Cu��4H����2 NO3-��Cu2����2 NO2����2H2O NO
���������������1����A��һ�ֻ�ɫ���ʹ��壬��A��S���ʣ�����B�Ƕ�������C������������B��C�Ļ�ѧ����ʽΪ2SO2+O2 2SO3����2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ��������������տ����е�ˮ����2Na2O2+2H2O=4NaOH+O2�����õ�NaOH��������CO2��������ӦΪ��CO2��2NaOH=Na2CO3��H2O;��Һ��ˮ���������γ�Na2CO3��10H2O������绯�õ�Na2CO3������ʱ������Ӧ��2NaHCO3
2SO3����2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ��������������տ����е�ˮ����2Na2O2+2H2O=4NaOH+O2�����õ�NaOH��������CO2��������ӦΪ��CO2��2NaOH=Na2CO3��H2O;��Һ��ˮ���������γ�Na2CO3��10H2O������绯�õ�Na2CO3������ʱ������Ӧ��2NaHCO3 Na2CO3��CO2����H2O.n(H2CO3)= 10g-9.38g=0.62g��62g/mol=0.01mol.����n(NaHCO3)=0.02mol��m (NaHCO3)=0.02mol��84g/mol=1.68g
Na2CO3��CO2����H2O.n(H2CO3)= 10g-9.38g=0.62g��62g/mol=0.01mol.����n(NaHCO3)=0.02mol��m (NaHCO3)=0.02mol��84g/mol=1.68g
m(Na2CO3)=10g-1.68g=8.32g�����Na2CO3����������Ϊ(8.32g��10g)��100%=83.2%��
��3����AΪ��ʹʪ��ĺ�ɫʯ����ֽ���������壬��AӦ���ǰ�����BΪNO��C�Ǻ���ɫ���壬ӦΪNO2����AΪ��������������ˮ�����Բ�������ˮ���ռ������ڰ������ܶȱȿ���С�����������ſ������ռ������鰱���Ƿ��ռ������ɽ�ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ�����������ɫ�ķ�̪��ֽ�����Թܿڴ�������ֽ��죬��֤���������ռ�����Ҳ������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�������ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ����������Ӧ�ķ���ʽΪCa(OH)2��2NH4Cl CaCl2��2NH3����2H2O.��n��NH4Cl��=
CaCl2��2NH3����2H2O.��n��NH4Cl��= =0.1mol����n��NH3��=0.1mol��V��NH3��="0.1mol" ��22.4L/mol=2.24L��
=0.1mol����n��NH3��=0.1mol��V��NH3��="0.1mol" ��22.4L/mol=2.24L��
��CΪNO2����ˮ��Ӧ���������NO����Ӧ�ķ���ʽΪ3NO2+H2O=2HNO3+NO��HNO3��ǿ����ʣ�����ǿ�����ԣ�����ͭ��Ӧ����Ӧ�����ӷ���ʽΪCu+4H++2NO3-=Cu2++2NO2��+2H2O��ʵ����Ϻ��Թ����ռ������������Ҫ�ɷ�ΪNO��
���㣺���ʵ��ƶϡ���Ԫ�صĵ��ʼ���������ת��������������ʺ����ļ��㡢������ʵ������ȡ���ռ������顢���ʡ���ѧ����ʽ�����ӷ���ʽ����д��֪ʶ��

��100mL18mol/L�������м�������ͭƬ�����Ȳ���ַ�Ӧ�������й�˵����ȷ����
| A����ַ�Ӧ��ת��1.8mol���� | B��H2SO4ֻ�������� |
| C����������Ӧ����H2 | D�����ĵ�ͭ������һ������57.6g |