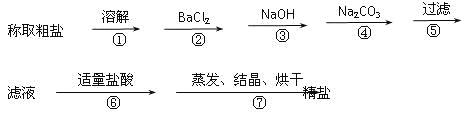
��Ŀ����
����Ŀ����Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�ĺ��������ǵ���������ٵ��Ͼ����⣬�ش��������⣺
��1���Ҵ���C2H5OH����δ����ȼ������ѡ������Һ��ȼ�ϡ�1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ__________��
��2������C3H8(g)= C3H6(g)+H2(g) H=+bkJmol��b��0���ķ�Ӧ�У���Ӧ����е�������________������������������������С��������������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ________�������ų�����������������������ת��Ϊ�����
��3��������ˮ��ȡ������Դ�����������о�������ȷ����________��
A ���ˮ����������ǿ���ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B �跨��̫����۽����������£�ʹˮ�ֽ��������
C Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ͬʱ�ͷ�����
D Ѱ��������������ڿ���������Դ���Էֽ�ˮ��ȡ����
��4����֪���������Ȼ�ѧ����ʽ��
A 2H2(g)+O2(g)=2H2O(l) H = -571.6kJmol-1
B C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) H= -2220kJmol-1
�ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽΪ________����A��B�����������22.4L��C3H8��H2������壨����H2���������Ϊ1/2������������������ȫȼ�գ���ų�������Ϊ________kJ��
���𰸡�C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1 С�� ���� AC B 1252.9kJ
��������
��1����֪1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ����������1mol�Ҵ�ȼ�շų�46a kJ��������
��2����Ӧ��������С����������������Ϊ���ȷ�Ӧ��
��3��A. ������ȼ�գ���ˮ�ֽ�����������
B. ̫����۽����������£������Ļ�ʯ��Դ����ʹˮ�ֽ����������
C. ˮ�ֽ���Ҫ����������
D. Ѱ��������������ͳɱ���
��4��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų���������һ��C��CO2(g)��S��SO2(g)��H��H2O(l)���ȼ�������22.4L��C3H8��H2���������C3H8�����ʵ�����H2�����ʵ�����Ϊ0.5mol���ٽ���Ȼ�ѧ����ʽ����ų���������
��1����֪1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ����������1mol�Ҵ�ȼ�շų�46a kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1��
�ʴ�Ϊ��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1��
��2����֪��H=+bkJmol1(b>0)���÷�ӦΪ���ȷ�Ӧ����Ӧ����е�������С����������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ������������ת��Ϊ�����
�ʴ�Ϊ��С�ڣ����գ�
��3��A. ������ȼ�գ���ˮ�ֽ��������������ֽⲻ�ܵõ���������A����
B. ̫����۽����������£������Ļ�ʯ��Դ����ʹˮ�ֽ������������B��ȷ��
C. ˮ�ֽ���Ҫ���������������ͷ���������C����
D. Ѱ��������������ͳɱ������ڿ���������Դ���Էֽ�ˮ��ȡ��������D��ȷ��
�ʴ�Ϊ��AC��
��4��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų���������һ��C��CO2(g)��S��SO2(g)��H��H2O(l)��A������Ϊ2mol��B��C3H8Ϊ1mol�������ɶ�����̼�����Һ̬ˮ�����ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽ��ΪB�������22.4L��C3H8��H2������壬��1mol������H2���������Ϊ1/2����C3H8�����ʵ�����H2�����ʵ�����Ϊ0.5mol������������������ȫȼ�գ��ų�������Ϊ![]() ��
��
�ʴ�Ϊ��B��1252.9kJ��

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�