��Ŀ����
�����£����ⶨij��Һ������ֻ��Na+��CH3COO-��H+��OH-���֣�������Ũ�ȴ�С������˳��Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-��������ܵ������ǣ�������
| A������Һ����ˮ�����c��H + �� һ��С�� 10 -7 mol/L |
| B������Һ��0.1 mol?L-1��CH3COOH��Һ������ʵ���Ũ�ȡ��������NaOH��Һ��϶��� |
| C������Һ�е����ʿ�����CH3COOH��CH3COONa |
| D����������Һ�м����������ᣬ��ʹ��Һ������Ũ�ȸı�Ϊc��CH3COO-����c��H+����c��Na+����c��OH-�� |
���㣺����Ũ�ȴ�С�ıȽ�
ר�⣺�����ˮ��ר��
��������Һ������Ũ�ȴ�С˳����c��CH3COO-����c��Na+����c��H+����c��OH-������Һ�����ԣ�����Һ�д���c��CH3COO-����c��Na+������������Ǵ����ƣ�����Һ�ʼ��ԣ�ʵ������Һ�����ԣ�����Һ�е�����ΪCH3COOH��CH3COONa��
A����������ˮ���룬���������ӵ��δٽ�ˮ���룻
B�������ʵ����Ĵ����NaOHǡ�÷�Ӧ���ɴ����ƣ���������Һ�ʼ��ԣ�
C������Һ�е�����ΪCH3COOH��CH3COONa��
D����������Һ�д���Ũ��ԶԶ���ڴ�����Ũ�ȣ����ܳ���c��CH3COO-����c��H+����c��Na+����c��OH-����
A����������ˮ���룬���������ӵ��δٽ�ˮ���룻
B�������ʵ����Ĵ����NaOHǡ�÷�Ӧ���ɴ����ƣ���������Һ�ʼ��ԣ�
C������Һ�е�����ΪCH3COOH��CH3COONa��
D����������Һ�д���Ũ��ԶԶ���ڴ�����Ũ�ȣ����ܳ���c��CH3COO-����c��H+����c��Na+����c��OH-����
���
�⣺��Һ������Ũ�ȴ�С˳����c��CH3COO-����c��Na+����c��H+����c��OH-������Һ�����ԣ�����Һ�д���c��CH3COO-����c��Na+������������Ǵ����ƣ�����Һ�ʼ��ԣ�ʵ������Һ�����ԣ�����Һ�е�����ΪCH3COOH��CH3COONa��
A����������ˮ���룬���������ӵ��δٽ�ˮ���룬��Һ�д������̶ȴ��ڴ��������ˮ��̶ȣ�������ˮ���룬��A����
B�������ʵ����Ĵ����NaOHǡ�÷�Ӧ����CH3COONa��CH3COONa��ǿ�������Σ����������ˮ�����Һ�ʼ��ԣ���B����
C��ͨ�����Ϸ���֪������Һ�е�����һ��ΪCH3COOH��CH3COONa����C����
D����������Һ�д���Ũ��ԶԶ���ڴ�����Ũ�ȣ�������Һ��c��H+����c��Na+�������Կ��ܳ���c��CH3COO-����c��H+����c��Na+����c��OH-������D��ȷ��
��ѡD��
A����������ˮ���룬���������ӵ��δٽ�ˮ���룬��Һ�д������̶ȴ��ڴ��������ˮ��̶ȣ�������ˮ���룬��A����
B�������ʵ����Ĵ����NaOHǡ�÷�Ӧ����CH3COONa��CH3COONa��ǿ�������Σ����������ˮ�����Һ�ʼ��ԣ���B����
C��ͨ�����Ϸ���֪������Һ�е�����һ��ΪCH3COOH��CH3COONa����C����
D����������Һ�д���Ũ��ԶԶ���ڴ�����Ũ�ȣ�������Һ��c��H+����c��Na+�������Կ��ܳ���c��CH3COO-����c��H+����c��Na+����c��OH-������D��ȷ��
��ѡD��
���������⿼������Ũ�ȴ�С�Ƚϣ�������Һ������Ũ��ȷ����Һ�������ǽⱾ��ؼ���֪���κε������Һ�ж����ڵ���غ�������غ㣬����Ũ�ȴ�С�Ƚϵ�ϰ������غ�˼���ɣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A����ϵ��������ܶȷ����仯��˵����Ӧû�дﵽƽ��״̬ |
| B����ϵ�и���ֵ�������������仯��˵��ƽ�ⷢ�����ƶ� |
| C����S��0�ķ�Ӧһ�����Է���Ӧ |
| D����Ӧ�ﵽƽ��״̬ʱ�������淴Ӧ���ʾ�Ϊ0 |
ʵ���е����в�����ȷ���ǣ�������
| A�����Թ�ȡ��Na2CO3��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� |
| B��Ba��NO3��2 ����ˮ���ɽ�����Ba��NO3��2 �ķ�Һ����ˮ���У�����ˮ������ˮ�� |
| C������������ʹNaCl ����Һ������ʱ��Ӧ����������NaCl ��Һȫ���������� |
| D����ҩ����ֽ�۰ѷ�ĩ״ҩƷ�����Թܵĵײ� |
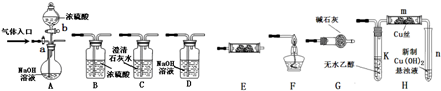
 �ⶨһ����������п�Ͻ���ǿ����Һ��Ӧ�����������������������úϽ�������п��������������������ʵ����Ʒ��800mL�ձ���100mL��Ͳ���̾�©����ͭ������п�Ͻ���Ʒ��Ũ���ᣨ�ܶ�1.19g/L����ˮ����ͼʾװ�ý���ʵ�飬�ش��������⣮����Ͻ���Ʒ��ȫ��Ӧ���������������������100mL��
�ⶨһ����������п�Ͻ���ǿ����Һ��Ӧ�����������������������úϽ�������п��������������������ʵ����Ʒ��800mL�ձ���100mL��Ͳ���̾�©����ͭ������п�Ͻ���Ʒ��Ũ���ᣨ�ܶ�1.19g/L����ˮ����ͼʾװ�ý���ʵ�飬�ش��������⣮����Ͻ���Ʒ��ȫ��Ӧ���������������������100mL��