��Ŀ����
19��NiԪ�ػ��������������зdz���Ҫ��Ӧ�ã�����NiO�����Ʊ�������������NiOOH���������ε�ص���Ҫ���ϣ�����NiSO4Ϊԭ����������NiO��NiOOH������ͼ1��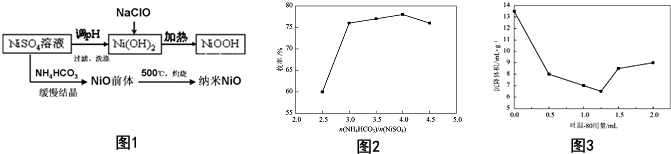
��1���Ʊ�NiOOH�����У�NiSO4��Һ���Ʒ�����NiSO4��������ϡ�����У���ϡ����һ��Ũ�ȣ����ˡ�ϴ�Ӻõ�Ni��OH��2���壬���֤��Ni��OH��2�Ѿ���ȫϴ���ýྻ��С�Թ�ʢ�����һ��ϴ��Һ���μ�BaCl2��Һ����û�л��dz���ʱ��˵�������Ѿ�ϴ����NaClO����Ni��OH��2�����ӷ���ʽΪClO-+2Ni��OH��2�TCl-+2NiOOH+H2O��
��2����֪Ksp[Ni��OH��2]=2��10-15�������£�������һ���� NaOH����ʹ1L ����0.001mol•L-1��NiSO4��0.0001mol•L-1��H2SO4��Һ�в���c��Ni2+����2��10-7 mol•L-1�����ָ������£��������NaOH�Ĺ�����������Ϊ0.092 g��
��3��NH3•H2O��Ũ�ȶ�����NiO�IJ��ʲ����ܴ�Ӱ�죮ͼ2ΪNiSO4�����ʵ���һ��ʱ����ͬ�ķ�Ӧ����ȶ��������������ʵ�Ӱ�죮����ͷ�Ӧ��NH4HCO3��NiSO4 �����ʵ�������2.5��4.0ʱ���������ߵ�ԭ��HCO3-��NH4+ˮ����ٽ���������NH4HCO3��Ũ������NH3•H2O��Ũ��Ҳ��֮��������������NiO�����ɣ�
��4���Ʊ����� NiO ʱ������һЩ������ˮ���л���磺����-80�����Ƶø����ʵ����ײ��ϣ�ԭ��������-80���Ӱ�����ǰ��������棬��ֹ�˿����žۣ��ۼ������Ӷ������ᾧ�õ�������С���ȵ�NiOǰ�壮
��5����������dz�ϸ�����һ����Ҫ��������������Һ���з�ɢ�Ժã�����������С����������ɢ�Խϲ��������������������ϴ�ͼ3������-80 �ļ�������ǰ����Һ��ʯ���г�������Ĺ�ϵ���ߣ�
ͨ��ͼ3����������-80����Ѽ�����Ϊ1.25 mL��
��6��NiOOH���Ʊ����ӵ�ص�ԭ�ϣ�ij���ӵ�ص��ܷ�ӦΪCd+2NiOOH+2H2O $?_{���}^{�ŵ�}$Cd��OH��2+2Ni��OH��2
�õ�طŵ�ʱ�����缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH-��
���� ��1������NiSO4��ҺʱҪ��ֹ������ˮ�⣬���Լ�������ϡ���ᣬͨ�������������Ƿ��������������֤��Ni��OH��2�Ѿ���ȫϴ����NaClO����Ni��OH��2���������Ӻ�NiOOH�����ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��2��ͨ��Ksp[Ni��OH��2]�ɼ����c��Ni2+����2��10-7 mol•L-1ʱ��Һ�����������ӵ�Ũ�ȣ��ݴ˼������NiSO4��Ӧ���������Ƶ����ʵ�����������������ʵ����ɼ���������ᷴӦ���������Ƶ����ʵ���������֮�ͼ�Ϊ�������NaOH�Ĺ�������ʵ������ټ����������
��3��HCO3-��NH4+ˮ����ٽ���������NH4HCO3��Ũ������NH3•H2O��Ũ��Ҳ��֮��������������NiO�����ɣ����Է�Ӧ��NH4HCO3��NiSO4 �����ʵ�������2.5��4.0ʱ���������ߣ�
��4���Ʊ����� NiO ʱ������һЩ������ˮ���л���磺����-80��������-80 ���Ӱ�����ǰ��������棬��ֹ�˿����žۣ��ۼ������Ӷ������ᾧ�õ�������С���ȵ�NiOǰ�壬�������Ƶø����ʵ����ײ��ϣ�
��5������ͼ3��֪������-80����Ѽ�����Ϊ1.25mLʱ��ǰ����Һ��ʯ���г��������С��
��6���������ӵ�ص��ܷ�ӦCd+2NiOOH+2H2O $?_{���}^{�ŵ�}$Cd��OH��2+2Ni��OH��2��֪���ŵ�ʱ��Cd�ǻ�ԭ�����ڸ�������������Ӧ��NiOOH����������������������ԭ��Ӧ���ݴ���д�缫��Ӧʽ��
��� �⣺��1������NiSO4��ҺʱҪ��ֹ������ˮ�⣬���Լ�������ϡ���ᣬ����NiSO4��Һ���Ʒ����ǽ�NiSO4��������ϡ�����У���ϡ����һ��Ũ�ȣ�����Ni��OH��2�Ѿ���ȫϴ���ķ������ýྻ��С�Թ�ʢ�����һ��ϴ��Һ���μ�BaCl2��Һ����û�л��dz���ʱ��˵�������Ѿ�ϴ����NaClO����Ni��OH��2���������Ӻ�NiOOH����Ӧ�����ӷ���ʽΪClO-+2Ni��OH��2�TCl-+2NiOOH+H2O��
�ʴ�Ϊ����NiSO4��������ϡ�����У���ϡ����һ��Ũ�ȣ��ýྻ��С�Թ�ʢ�����һ��ϴ��Һ���μ�BaCl2��Һ����û�л��dz���ʱ��˵�������Ѿ�ϴ����ClO-+2Ni��OH��2�TCl-+2NiOOH+H2O��
��2��ͨ��Ksp[Ni��OH��2]�ɼ����c��Ni2+����2��10-7 mol•L-1ʱ��Һ�����������ӵ�Ũ��Ϊ1��10-4 mol•L-1��������NiSO4��Ӧ���������Ƶ����ʵ���Ϊ2��1L��0.001mol•L-1+1L��1��10-4 mol•L-1=0.0021mol��������������ʵ����ɼ���������ᷴӦ���������Ƶ����ʵ���Ϊ0.0002mol�����Լ����NaOH�Ĺ�������ʵ�������Ϊ0.0021mol+0.0002mol=0.0023mol��������Ϊ����0.092g��
�ʴ�Ϊ��0.092��
��3��HCO3-��NH4+ˮ����ٽ���������NH4HCO3��Ũ������NH3•H2O��Ũ��Ҳ��֮��������������NiO�����ɣ����Է�Ӧ��NH4HCO3��NiSO4 �����ʵ�������2.5��4.0ʱ���������ߣ�
�ʴ�Ϊ��HCO3-��NH4+ˮ����ٽ���������NH4HCO3��Ũ������NH3•H2O��Ũ��Ҳ��֮��������������NiO�����ɣ�
��4���Ʊ����� NiO ʱ������һЩ������ˮ���л���磺����-80��������-80 ���Ӱ�����ǰ��������棬��ֹ�˿����žۣ��ۼ������Ӷ������ᾧ�õ�������С���ȵ�NiOǰ�壬�������Ƶø����ʵ����ײ��ϣ�
�ʴ�Ϊ������-80 ���Ӱ�����ǰ��������棬��ֹ�˿����žۣ��ۼ������Ӷ������ᾧ�õ�������С���ȵ�NiOǰ�壻
��5������ͼ3��֪������-80����Ѽ�����Ϊ1.25mLʱ��ǰ����Һ��ʯ���г��������С��
�ʴ�Ϊ��1.25��
��6���������ӵ�ص��ܷ�ӦCd+2NiOOH+2H2O $?_{���}^{�ŵ�}$Cd��OH��2+2Ni��OH��2��֪���ŵ�ʱ��Cd�ǻ�ԭ�����ڸ�������������Ӧ��NiOOH����������������������ԭ��Ӧ���缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH-��
�ʴ�Ϊ��NiOOH+H2O+e-=Ni��OH��2+OH-��
���� ���⿼�������Ʊ�������������ɵIJⶨ��ԭ��������ԭ������Ŀ�ۺ���ǿ���Ƕ�ѧ���ۺ������Ŀ��飬��ȷԭ���ǹؼ����Ѷ��еȣ�

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�| ѡ�� | ���������� | ��Һ |
| A | ͨ��CO2����Һ����ǣ�����ͨ��CO2����������Һ����� | BaCl2��Һ |
| B | ͨ��CO2����Һ����ǣ�����ͨCO2��������������ʧ | Na2SiO3��Һ |
| C | ͨ��CO2����Һ����ǣ��ټ���Ʒ����Һ����ɫ��ȥ | Ca��ClO��2��Һ |
| D | ͨ��CO2����Һ����ǣ�����ͨCO2��������������ʧ���ټ�������NaOH��Һ�������Ա仯 | Ca��OH��2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Al��AlO2- | B�� | CO2��HCO3- | C�� | SiO2��H2SiO3 | D�� | SO2��H2SO3 |
H2O��g���TH2O ��l����H2=-44kJ•mol-1
2H2O2��l���T2H2O��l��+O2��g����H3=-196.4kJ•mol-1
��������������ⷴӦ���Ȼ�ѧ����ʽ�ɱ�ʾΪ��������
| A�� | N2H4��g��+2H2O2��l���TN2��g��+4H2O��l����H=+817.63 kJ•mol-1 | |
| B�� | N2H4��g��+2H2O2��l���TN2��g��+4H2O��g����H=-641.63 kJ•mol-1 | |
| C�� | N2H4��g��+2H2O2��l���TN2��g��+4H2O��l����H=-641.63 kJ•mol-1 | |
| D�� | N2H4��g��+2H2O2��l���TN2��g��+4H2O��g����H=-817.63 kJ•mol-1 |
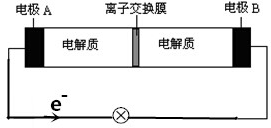
| A�� | �ŵ�ʱ��Na+���ҵ���ͨ�����ӽ���Ĥ | |
| B�� | �ŵ�ʱ��������ӦʽΪ3NaBr-2e-�TNaBr3+2Na+ | |
| C�� | ���ʱ��A��Ӧ��ֱ����Դ�������� | |
| D�� | �ŵ�ʱ������0.1molNa+ͨ�����ӽ���Ĥʱ��B������0.3molNaBr���� |
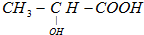 �������������������·ֽ�ΪCO2��H2O��
�������������������·ֽ�ΪCO2��H2O��


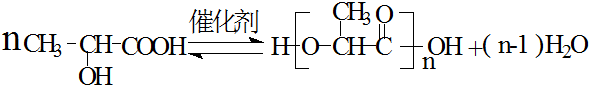 ��
�� $��_{��}^{-H_{20}}$CH3CH=CHCHO��ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
$��_{��}^{-H_{20}}$CH3CH=CHCHO��ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�