��Ŀ����
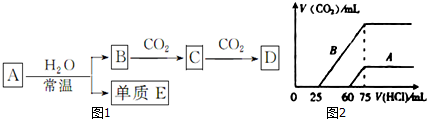
7�������ڷ�����ᴿ���ʵķ�����A�����ˡ�B��������C�����ȷֽ⡡D��ϴ���� E����ȡ��Һ�� F���ؽᾧ��
G����Һ�� H������ ����֪���ʵ���������������
���и�������ķ�����ᴿӦѡ���������ַ�������ʣ�������ĸ��
��1����ȥCa��OH��2��Һ��������CaCO3��������A��
��2����ȥO2��������ˮ��������D��
��3����ȥ������л��е�����NaI����B��
��4����ȥ�������е�CaCO3����C��
��5��������غ��Ȼ��ƵĻ��Һ�л���������F��
���� ��1��̼��Ʋ�����ˮ��
��2������Ũ�������������
��3���������������
��4��̼��Ƽ��ȷֽ�����CaO��
��5������غ��Ȼ����ܽ�����¶�Ӱ�첻ͬ��
��� �⣺��1�����ù��˵ķ�����ȥCa��OH��2��Һ��������CaCO3�����ʴ�Ϊ��A��
��2����ȥO2��������ˮ��������ʢ��Ũ�����ϴ��ƿϴ�����ӣ��ʴ�Ϊ��D��
��3����������������������������õ��⣬�ʴ�Ϊ��B��
��4��̼��Ƽ��ȷֽ����������ƣ����ü��ȷֽⷨ���룬�ʴ�Ϊ��C��
��5������غ��Ȼ����ܽ�����¶�Ӱ�첻ͬ���������ؽᾧ��������غ��Ȼ��ƵĻ��Һ�л������أ��ʴ�Ϊ��F��
���� ���⿼�����ʵķ��롢�ᴿ������ѡ��Ϊ��Ƶ���㣬�������ʵ����ʼ�����ԭ��Ϊ���Ĺؼ���ע���������ʲ��켰���뷽����ѡ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
17���������仯����ת����ϵ�ǻ�ѧѧϰ����Ҫ����֮һ�����и������ʵ�ת����ϵ����ȫ����ͨ��һ����Ӧ��ɵ��ǣ�������
| A�� | Na��NaOH��Na2CO3��NaCl | B�� | Al��Al2O3��Al��OH��3��AlCl3 | ||
| C�� | Mg��MgCl2��Mg��OH��2��MgSO4 | D�� | Fe��FeCl2��Fe��OH��2��Fe��OH��3 |
18����֪�����ӵ�����NA������˵����ȷ���ǣ�������
| A�� | 16g CH4�к���4NA��ԭ�� | |
| B�� | 1mol/L NaCl��Һ����NA��Na+ | |
| C�� | 1mol Al������ϡ���ᷴӦת��3NA������ | |
| D�� | ��״���£�22.4L CCl4�к���4NA����ԭ�� |
12����ʵ��ʱ��С��ʹƤ����մ��һЩ������أ��γɵĺڰߺܾò�������������ò��ᣨ�Ҷ��ᣩ��ϡ��Һϴ�ӣ��ڰ߿���Ѹ����ȥ�������ӷ���ʽΪ��MnO4-+H2C2O4+H+�TCO2��+Mn2++�������й���������ȷ���ǣ�?��
| A�� | �÷�Ӧ��������ΪKMnO4 | |
| B�� | ������ԭ��Ӧ����H2C2O4 | |
| C�� | �����ӷ���ʽ�Ҳ���ڵIJ�����H2O | |
| D�� | 6mol H+�μӷ�Ӧʱ������ת��10mol |
16������������䣮����˵����ȷ���ǣ�������
| A�� | 2NO2��g��?N2O4��g��������ʱ��ϵ��ɫ����˵������ӦΪ���ȷ�Ӧ | |
| B�� | N2��g��+3H2��g��?2NH3��g����������ʱNH3��Ũ�ȼ�С����ƽ�ⳣ��K���� | |
| C�� | CO��g��+H2O��g��?CO2��g��+H2��g��������ʱCO��ת��������˵������ӦΪ���ȷ�Ӧ | |
| D�� | ��Ӧ2HI��g��?H2��g��+I2��g���ﵽƽ�������HI�����ʵ�����ƽ�ⲻ�ƶ� |

 C6H14
C6H14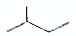 C5H12
C5H12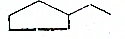 C7H14
C7H14 C10H8��
C10H8��