��Ŀ����
��16�֣�������ҵ�г����ұ�����ķ����Ʊ�����ϩ��
��1����֪ij�¶��£�
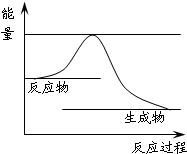
��Ӧ�٣�CO2��g�� +H2 ��g����CO��g�� + H2O��g������H�� +41.2 kJ/mol
��Ӧ�ڣ� ��g����
��g���� ��g��+H2��g������H=" +117.6" kJ/mol
��g��+H2��g������H=" +117.6" kJ/mol
�ڵĻ�ѧ��Ӧƽ�ⳣ���ֱ�ΪK1��K2��
��д��������̼�����ұ��Ʊ�����ϩ���Ȼ�ѧ��Ӧ����ʽ ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� ����K1��K2��ʾ����
��2�����ڷ�Ӧ�٣����º��������£����ܱ������м���2molCO2��2molH2������Ӧ�ﵽƽ�������˵����ȷ���� ��
��3���º��������£���Ӧ�ٴﵽƽ���t1ʱ��ͨ������CO2��������ͼ�л���t1֮������淴Ӧ���ߣ���������ע��

��4����֪ij�¶��£� Ag2SO4��M��312g/mol�����ܽ��Ϊ0.624g/100g H2O�����¶���Ksp��Ag2SO4���� ������λ��Ч���֣�
��5����ⷨ�Ʊ��������ƣ�Na2FeO4�����ܷ�ӦʽΪ��Fe+2H2O+2OH- �� FeO42-+3H2���������Һѡ��NaOH��Һ���õ������������� (д��ѧʽ) �������ĵ缫��ӦʽΪ�� ��
��1����֪ij�¶��£�
��Ӧ�٣�CO2��g�� +H2 ��g����CO��g�� + H2O��g������H�� +41.2 kJ/mol
��Ӧ�ڣ�
 ��g����
��g���� ��g��+H2��g������H=" +117.6" kJ/mol
��g��+H2��g������H=" +117.6" kJ/mol�ڵĻ�ѧ��Ӧƽ�ⳣ���ֱ�ΪK1��K2��
��д��������̼�����ұ��Ʊ�����ϩ���Ȼ�ѧ��Ӧ����ʽ ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� ����K1��K2��ʾ����
��2�����ڷ�Ӧ�٣����º��������£����ܱ������м���2molCO2��2molH2������Ӧ�ﵽƽ�������˵����ȷ���� ��
| A����Ϊ�÷�Ӧ�����ȷ�Ӧ�����������¶ȣ�����Ӧ���������淴Ӧ���ʼ�С�� |
| B������������1molCO2��1mol H2��ƽ��������Ӧ�����ƶ��� |
| C��������ͨ��1mol CO2��ƽ��������Ӧ�����ƶ���CO2��ת�������� |
| D��ѹ�������ƽ�ⲻ�ƶ�����Ӧ��Ͳ����Ũ�ȶ����䣻 |

��4����֪ij�¶��£� Ag2SO4��M��312g/mol�����ܽ��Ϊ0.624g/100g H2O�����¶���Ksp��Ag2SO4���� ������λ��Ч���֣�
��5����ⷨ�Ʊ��������ƣ�Na2FeO4�����ܷ�ӦʽΪ��Fe+2H2O+2OH- �� FeO42-+3H2���������Һѡ��NaOH��Һ���õ������������� (д��ѧʽ) �������ĵ缫��ӦʽΪ�� ��
��1��CO2��g��+ ��g����
��g���� ��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�
��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�
K��K1��K����2�֣� ��2��B��2�֣� (3) ��2�֣�
��2�֣�
��4��3.2��10-5 ��3�֣� ��5�� Fe Fe-6e+8OH- ��FeO42-+4H2O����2�֣�
 ��g����
��g���� ��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�
��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�K��K1��K����2�֣� ��2��B��2�֣� (3)
 ��2�֣�
��2�֣���4��3.2��10-5 ��3�֣� ��5�� Fe Fe-6e+8OH- ��FeO42-+4H2O����2�֣�
�����������1����֪��Ӧ�٣�CO2��g�� +H2 ��g����CO��g�� + H2O��g������H�� +41.2 kJ/mol����Ӧ�ڣ�
 ��g����
��g���� ��g��+H2��g������H=" +117.6" kJ/mol������ݸ�˹���ɿ�֪��+�ڼ��õ��Ȼ�ѧ����ʽCO2��g��+
��g��+H2��g������H=" +117.6" kJ/mol������ݸ�˹���ɿ�֪��+�ڼ��õ��Ȼ�ѧ����ʽCO2��g��+ ��g����
��g���� ��g��+ H2O��g�� ��H�� +158.8 kJ/mol����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����÷�Ӧ�Ļ�ѧƽ�ⳣ��
��g��+ H2O��g�� ��H�� +158.8 kJ/mol����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����÷�Ӧ�Ļ�ѧƽ�ⳣ��

��2��A�������¶ȣ�����Ӧ���������淴Ӧ����Ҳ����A����ȷ��B���¶ȡ�������䣬����������1molCO2��1mol H2����Ӧ��Ũ������ƽ��������Ӧ�����ƶ���B��ȷ��C��������ͨ��1mol CO2����Ӧ��Ũ������ƽ��������Ӧ�����ƶ�����CO2��ת���ʼ�С��C����ȷ��D����Ӧǰ��������䣬ѹ�������ƽ�ⲻ�ƶ�������Ӧ��Ͳ����Ũ�ȶ�����D����ȷ����ѡB��
��3���º��������£���Ӧ�ٴﵽƽ���t1ʱ��ͨ������CO2����Ӧ��Ũ����������Ӧ��������Ȼ����С���淴Ӧ����������ƽ��������Ӧ������У�����t1֮������淴Ӧ����Ϊ�����𰸣���
��4����֪ij�¶��£�Ag2SO4��M��312g/mol�����ܽ��Ϊ0.624g/100g H2O����100gˮ����Һ������ƿ�����100ml�������ʵ����ʵ�����0.624g��312g/mol��0.002mol����Ũ����0.02mol/L��������������ĵ��뷽��ʽ��֪��Һ��������Ũ�Ⱥ������Ũ�ȷֱ���0.04mol/L��0.02mol/L�����Ը��¶���Ksp��Ag2SO4����c2(Ag��)��c(SO42��)��0.042��0.02��3.2��10-5��
��5������������ʧȥ���ӣ�����������Ӧ�������õ����ӷ�����ԭ��Ӧ��������ܷ�ӦʽΪ��Fe+2H2O+2OH- ��FeO42-+3H2��֪������������Һѡ��NaOH��Һ���õ�������������Fe����ʧȥ���ӣ�ת��ΪFeO42-����������ĵ缫��ӦʽΪFe-6e+8OH- ��FeO42-+4H2O��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 HBr��HBrO�����д�ʩ������ʹ��Һ��ɫ��dz����
HBr��HBrO�����д�ʩ������ʹ��Һ��ɫ��dz����  2 FeO(s)+CO(g)
2 FeO(s)+CO(g) 2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־��
2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־�� 
 ���õ������������ݣ�
���õ������������ݣ�
 ____________
____________ ���<������>������=������
���<������>������=������

 ��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ1��10
��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ1��10 mol/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ__________mol/L��
mol/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ__________mol/L��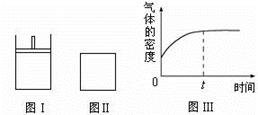 ����
���� a Z��g����
a Z��g����

 pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� ��
pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� �� CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ�� ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ�� ��