��Ŀ����
���ҹ�����ʳ���м������أ�KIO3���ķ�����ֹȱ������ļ�����
��1���������У��������� ��ѡ���������������Ԫ�أ�ȱ������ļ����� ��
��2����֪��������Һ��IO3-�ɺ�I-������Ӧ��IO3-+5I-+6H+=3I2+3H2O����ȡ��ˮ�еĵ�ʱ����ѡ�õ��Լ��� ������������Ӧ������ʳ���м������ر���ʹ�õ������� ���ɹ�ѡ�õ��У�������ˮ������ɫʯ����ֽ���۵⻯�ص�����ֽ���ܵ��ۣ���ʳ�ǣ���ʳ�ף���
��3����֪����������ֽ⣬���õ���ؼӵ��ν������ʱӦע�� ��
II�� ������������ʡ������Ⱦ��Ϊ���أ����������������ü�����⣮�����������pH��5.6����������������͵����������γ��������Ҫ���ʣ�
��1���������������pH��һ��ʱ���ڱ�СȻ���ȶ���ijһ��ֵ��ԭ����H2SO3�ܿ���Ӱ����ɵģ��仯ѧ����ʽΪ��
��2��Ϊ�˽�������������ɵĿ�����Ⱦ��һ�ַ������ں���ȼ�ϣ���ú��ȼ�չ����м�����ʯ�ң����ַ����С��ƻ������������ַ�����ȼ��ȼ�չ����еġ�����ӦΪ�� ��
III��������л�ѧ����ѧ������������������أ���ش�
��1������ѧ�ⶨ������ƽ������65%����̼18%������10%�����Ͼ�Ϊ������������������Ԫ����ԭ����Ŀ������ ����Ԫ�ط��ţ���
��2��Ϊ��֤ij�������ʵ���Ҫ�ɷ������ƻ������ƣ���������������֤��һ���� ���ɣ����û�ѧ������֤����ʹ�õĻ�ѧ�Լ�Ϊ ��
��3���ݱ�����ȫ����ÿ���������ʴ��ɵ�ֱ�Ӿ�����ʧԼ��7000����Ԫ���ҹ��������ʴ��ɵ���ʧռ����������ֵ��GNP����4%�������ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��ӦʽΪ ��
��4����Ȼ����Ҫ�ɷֵĽṹ��ʽ�� ������Ŀ����ʹ��������֮��ͨ�����Ž����������γ� ���Ӷ����������ܣ�
��1������������������
��2����֪��������Һ��IO3-�ɺ�I-������Ӧ��IO3-+5I-+6H+=3I2+3H2O����ȡ��ˮ�еĵ�ʱ����ѡ�õ��Լ���
��3����֪����������ֽ⣬���õ���ؼӵ��ν������ʱӦע��
II�� ������������ʡ������Ⱦ��Ϊ���أ����������������ü�����⣮�����������pH��5.6����������������͵����������γ��������Ҫ���ʣ�
��1���������������pH��һ��ʱ���ڱ�СȻ���ȶ���ijһ��ֵ��ԭ����H2SO3�ܿ���Ӱ����ɵģ��仯ѧ����ʽΪ��
��2��Ϊ�˽�������������ɵĿ�����Ⱦ��һ�ַ������ں���ȼ�ϣ���ú��ȼ�չ����м�����ʯ�ң����ַ����С��ƻ������������ַ�����ȼ��ȼ�չ����еġ�����ӦΪ��
III��������л�ѧ����ѧ������������������أ���ش�
��1������ѧ�ⶨ������ƽ������65%����̼18%������10%�����Ͼ�Ϊ������������������Ԫ����ԭ����Ŀ������
��2��Ϊ��֤ij�������ʵ���Ҫ�ɷ������ƻ������ƣ���������������֤��һ����
��3���ݱ�����ȫ����ÿ���������ʴ��ɵ�ֱ�Ӿ�����ʧԼ��7000����Ԫ���ҹ��������ʴ��ɵ���ʧռ����������ֵ��GNP����4%�������ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��ӦʽΪ
��4����Ȼ����Ҫ�ɷֵĽṹ��ʽ��
���㣺��Ԫ�ض����彡������Ҫ����,�����ĵ绯ѧ��ʴ�����,�ȡ��塢�⼰�仯������ۺ�Ӧ��,�����������Ⱦ������
ר�⣺��ѧӦ��
��������1����Ԫ�ذ��������ܡ�ͭ��п�������̡��⡢�����⡢����ȱ���ͯ��ͷ��С���������£���״���״��Ӳ��ȣ�
��2����IO3-+5I-+6H+�T3I2+3H2O��֪���÷�Ӧ��Ҫ������������Ҫ�����ӣ���ѡ���۵⻯����ֽ���飬�Դ˽��
��3�����������Ϣ���������ֽ⡱��������
II����1��H2SO3���������ԣ��ױ���������������ΪH2SO4��
��2��ú�к���S����������Ӧ����SO2��SO2��CaO��Ӧ����CaSO3����һ����Ӧ����CaSO4��
III����1�������������Ϊm���������и�Ԫ��ԭ�ӵ����ʵ���=m�����������¸�Ԫ�ص�Ħ��������
��2�����ܱ���������������������������Һ��Ӧ����������
��3�����Ի������������£���������������ʴ����������ʧ���ӷ���������Ӧ�������������õ��ӷ�����ԭ��Ӧ��
��4����Ȼ�����ӹ�����Եõ�����ȶ������������ӷ����˱仯�������˱�Ĵ�����л��
��2����IO3-+5I-+6H+�T3I2+3H2O��֪���÷�Ӧ��Ҫ������������Ҫ�����ӣ���ѡ���۵⻯����ֽ���飬�Դ˽��
��3�����������Ϣ���������ֽ⡱��������
II����1��H2SO3���������ԣ��ױ���������������ΪH2SO4��
��2��ú�к���S����������Ӧ����SO2��SO2��CaO��Ӧ����CaSO3����һ����Ӧ����CaSO4��
III����1�������������Ϊm���������и�Ԫ��ԭ�ӵ����ʵ���=m�����������¸�Ԫ�ص�Ħ��������
��2�����ܱ���������������������������Һ��Ӧ����������
��3�����Ի������������£���������������ʴ����������ʧ���ӷ���������Ӧ�������������õ��ӷ�����ԭ��Ӧ��
��4����Ȼ�����ӹ�����Եõ�����ȶ������������ӷ����˱仯�������˱�Ĵ�����л��
���
�⣺��1����������Ԫ�أ�ȱ�������ͯ��ͷ��С���������£���״���״��Ӳ��ȣ�
�ʴ�Ϊ��������ͯ��ͷ��С���������£���״���״��Ӳ��ȣ�
��2����IO3-+5I-+6H+�T3I2+3H2O��I2�����۱�����֪������ʳ���д���IO3-���÷�Ӧ��Ҫ������������Ҫ�����ӣ���ѡ���۵⻯����ֽ���飬���ɵ�I2�ɵ��ۼ��飬�ʴ�Ϊ���������Ȼ�̼�ȣ��ۢޣ�
��3����������Ϣ����������ֽ⣬��������ʱҪ�ȷ�����ʱ�ټ��Σ��ʴ�Ϊ������ʱ��Ҫ�ȷ��λ�����⿿����ǰ�ټ����Σ�
II����1��H2SO3���������ԣ��ױ���������������ΪH2SO4����Ӧ�ķ���ʽΪ2H2SO3+O2=2H2SO4��
�ʴ�Ϊ��2H2SO3+O2=2H2SO4��
��2��ú�к���S����������Ӧ����SO2��SO2��CaO��Ӧ����CaSO3����Ӧ����ʽΪCaO+SO2�TCaSO3����һ����Ӧ����CaSO4����Ӧ����ʽΪ2CaSO3+O2�TCaSO4����ȼ��ȼ�չ����еġ�����ӦΪ2CaO+2SO2+O2=2CaSO4���ʴ�Ϊ��2CaO+2SO2+O2=2CaSO4��
III����1�������������Ϊm���������и�Ԫ��ԭ�ӵ����ʵ���=m�����������¸�Ԫ�ص�Ħ������������
n��C��=
=0.012mmol��n��H��=
=0.1mmol��n��O��=
=0.041mmol
�ʴ�Ϊ��H��
��2�����ܱ����������������ܱ�����������������������Һ��Ӧ�����������ʴ�Ϊ�������Ƿ�������������������Һ��
��3�������ڳ�ʪ�Ŀ����з���������ʴ����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Fe-2e-=Fe2+���ʴ�Ϊ��Fe-2e-=Fe2+��
��4����Ȼ�ijɷ��Ǿ������ϩ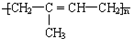 ����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��
����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��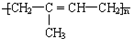 �� ��״�ṹ��
�� ��״�ṹ��
�ʴ�Ϊ��������ͯ��ͷ��С���������£���״���״��Ӳ��ȣ�
��2����IO3-+5I-+6H+�T3I2+3H2O��I2�����۱�����֪������ʳ���д���IO3-���÷�Ӧ��Ҫ������������Ҫ�����ӣ���ѡ���۵⻯����ֽ���飬���ɵ�I2�ɵ��ۼ��飬�ʴ�Ϊ���������Ȼ�̼�ȣ��ۢޣ�
��3����������Ϣ����������ֽ⣬��������ʱҪ�ȷ�����ʱ�ټ��Σ��ʴ�Ϊ������ʱ��Ҫ�ȷ��λ�����⿿����ǰ�ټ����Σ�
II����1��H2SO3���������ԣ��ױ���������������ΪH2SO4����Ӧ�ķ���ʽΪ2H2SO3+O2=2H2SO4��
�ʴ�Ϊ��2H2SO3+O2=2H2SO4��
��2��ú�к���S����������Ӧ����SO2��SO2��CaO��Ӧ����CaSO3����Ӧ����ʽΪCaO+SO2�TCaSO3����һ����Ӧ����CaSO4����Ӧ����ʽΪ2CaSO3+O2�TCaSO4����ȼ��ȼ�չ����еġ�����ӦΪ2CaO+2SO2+O2=2CaSO4���ʴ�Ϊ��2CaO+2SO2+O2=2CaSO4��
III����1�������������Ϊm���������и�Ԫ��ԭ�ӵ����ʵ���=m�����������¸�Ԫ�ص�Ħ������������
n��C��=
| m��18% |
| 12 |
| m��10% |
| 1 |
| m��65% |
| 16 |
�ʴ�Ϊ��H��
��2�����ܱ����������������ܱ�����������������������Һ��Ӧ�����������ʴ�Ϊ�������Ƿ�������������������Һ��
��3�������ڳ�ʪ�Ŀ����з���������ʴ����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Fe-2e-=Fe2+���ʴ�Ϊ��Fe-2e-=Fe2+��
��4����Ȼ�ijɷ��Ǿ������ϩ
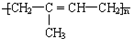 ����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��
����Ȼ�������Խṹǿ�Ⱥ����Բ��S�������ã�ʹ���ṹ����״�������״��������нϸߵ�ǿ�ȡ����Ժͻ�ѧ�ȶ��ԣ��ʴ�Ϊ��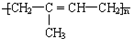 �� ��״�ṹ��
�� ��״�ṹ��
������������Ҫ��������Ԫ�ء����ʼ��顢������ʴ����Ȼ�ijɷֵ�֪ʶ�����ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���и������ʣ���Ϊͬ���칹����ǣ�������
| A��O2��O3 | ||||
B��
| ||||
C�� | ||||
D�� |
���л�ѧ��Ӧ�������ȷ�Ӧ���ǣ�������
| A������������Һ�����ᷴӦ |
| B��Ũ��������ˮ |
| C��þ��ϡ���ᷴӦ |
| D�����������������Ȼ�炙�� |
��ݮ����������pHΪ5.6��6.0�����������У����з��ϲ��ʺ�ʩ���ڲݶ�������ǣ�������
| A����ˮ | B������� |
| C���Ȼ��� | D������ |



