��Ŀ����
17����һ������Һ�����ܺ���NH4+��H+��K+��Mg2+��Al3+��Fe3+��I-��CO32-��SO42-��AlO2-��ijͬѧȡ����Һ��������ʵ�飺����PH��ֽ���飬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ���μӹ�������������a����Һ�����Ա�Ϊ���ԣ�b����Һ����������c��������ȫ�ܽ⣻d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ�����ش��������⣮
��1���ɢٿ���֤��H+ �Ĵ��ڣ��ų�CO32-��AlO2- �Ĵ��ڣ�
��2���ɢڿ���֤��I- �Ĵ��ڣ�ͬʱ�ų�Fe3+ �Ĵ��ڣ�
��3���ɢۿ���֤��Al3+��NH4+ �Ĵ��ڣ�û��Mg2+ �Ĵ��ڣ�
���� ����pH��ֽ��⣬��Һ��ǿ���ԣ�˵����Һ�д���H+���������ӹ����֪һ��������CO32-��AlO2-��
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��˵����Һ��һ������I-���������ӹ�������жϿ�֪��һ��������Fe3+��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ��к��
b����Һ����������������������ֻ�������ӳ�����
c��������ȫ�ܽ⣬֤�����ɵij���������������ԭ��Һ��һ������Al3+��
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������֤�����ɵ������ǰ�����ԭ��Һ��һ������笠����ӣ��ɴ˷������
��� �⣺����pH��ֽ��⣬��Һ��ǿ���ԣ�˵����Һ�д���H+���������ӹ����֪һ��������CO32-��AlO2-��
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��˵����Һ��һ������I-���������ӹ�������жϿ�֪��һ��������Fe3+��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ��к��
b����Һ����������������������ֻ�������ӳ�����
c��������ȫ�ܽ⣬֤�����ɵij���������������ԭ��Һ��һ������Al3+��
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������֤�����ɵ������ǰ�����ԭ��Һ��һ������笠����ӣ�
��1���ɢ���pH��ֽ��⣬��Һ��ǿ���ԣ�˵����Һ�д���H+�������ų�CO32-��AlO2-�Ĵ��ڣ��ʴ�Ϊ��H+��CO32-��AlO2-��
��2���ɢڿ���֤��I-һ���Ĵ��ڣ�CCl4����ֵⵥ�ʵ���ɫ֤����I-��Fe3+�ڸû�������I-���ܹ��棬�������ӹ���ͬʱ�ų�Fe3+�Ĵ��ڣ�
�ʴ�Ϊ��I-��Fe3+��
��3����֤����Һ��һ������Al3+��NH4+��û��Mg2+�Ĵ��ڣ��ʴ�Ϊ��Al3+��NH4+��Mg2+��
���� ���⿼�������Ӽ����ʵ�鷽���ͷ�Ӧ������жϣ��������ʺ����ӷ�Ӧ���е����ӹ�������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | NH4Cl�ĵ���ʽ�� | |
| B�� | �����Ľṹʽ����N��N�� | |
| C�� | �Ȼ�����ӵ��γɹ��̿��õ���ʽ��ʾʽ�� | |
| D�� | ��ˮ�Ļ�ѧʽΪ 21H2O����D2O�� |
| A�� |  +HNO3$��_{��}^{Ũ����}$ +HNO3$��_{��}^{Ũ����}$  +H2O��ȡ����Ӧ +H2O��ȡ����Ӧ | |
| B�� | CH2�TCH2+Br2��CH2Br-CH2Br���ӳɷ�Ӧ | |
| C�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��ȡ����Ӧ | |
| D�� | CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��������ӦҲ����ȡ����Ӧ |
| A�� | ��������������� | B�� | ����ԭ����������� | ||
| C�� | ������������� | D�� | ������ԭ������� |
| A�� | ʯ�͵Ĵ�������ú�ĸ�������Եõ���������˵��ʯ�ͺ�ú�к��з����� | |
| B�� | ʯ���ѽ��Ŀ����Ҫ��Ϊ�˵õ���������� | |
| C�� | ʯ�ͷ���õ��IJ����������ȡ��ˮ�е��� | |
| D�� | ʯ���ѻ���Ҫ�õ�������ϩ����ϩ����̬�� |
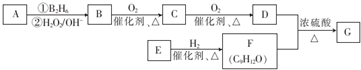
 ���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ��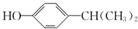 ��
��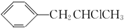 Ϊԭ��Ҳ�ɺϳ�F����ο���Ŀ�е������Ϣд����Ӧ�ĺϳ�·��ͼ����Ӧ�����е��Լ�д�ڼ�ͷ�Ϸ�������д�ڼ�ͷ�·�����
Ϊԭ��Ҳ�ɺϳ�F����ο���Ŀ�е������Ϣд����Ӧ�ĺϳ�·��ͼ����Ӧ�����е��Լ�д�ڼ�ͷ�Ϸ�������д�ڼ�ͷ�·�����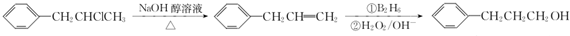 ��
��