��Ŀ����
ij������ȤС��Ϊ�ⶨά����C��̼���������������ȡά����C��Ʒ���飬�Ƶ�����0.352g������ȼ�չ��У���ͼ��������ͨ�����������þƾ���Ƴ���������Ʒ�����������Ⱥ�ͨ��A��Bװ�ã����߷ֱ�����0.144g��0.528g��

�Իش��������⣺
��1���������߿��ڵ�װ��A����ע��ʢװ�Լ���
��2��ʵ���������������ã���ȷ��ά����C��ĩ��ַ�Ӧ���� ��
��3��ά����C��̼������������ ������������� ��
��4��ά����C���Ƿ�����Ԫ�أ�Ϊʲô������ͨ������˵����
��5�������Ҫ��ȷ��ά����C�ķ���ʽ���㻹��Ҫ��Щ��Ϣ��

�Իش��������⣺
��1���������߿��ڵ�װ��A����ע��ʢװ�Լ���
��2��ʵ���������������ã���ȷ��ά����C��ĩ��ַ�Ӧ����
��3��ά����C��̼������������
��4��ά����C���Ƿ�����Ԫ�أ�Ϊʲô������ͨ������˵����
��5�������Ҫ��ȷ��ά����C�ķ���ʽ���㻹��Ҫ��Щ��Ϣ��
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺
��������1���ⶨά����C��̼�������������������װ��A�������յõ���ˮ�������ⶨ��Ԫ�غ�����
��2��ʵ���������������ã�ȷ��ά����C��ĩ��ַ�Ӧ��ͬʱ�����ɵĶ�����̼��ˮ����ȫ������װ��AB��ȫ�����գ�
��3���л���ͨ���������������ȼ�����ɶ�����̼��ˮ�����������Ⱥ�ͨ��Ũ����ͼ�ʯ�ң����߷ֱ�����0.144g��0.528g����ȼ������ˮΪ0.144g��������̼Ϊ0.528g�����������غ�����ά����C��̼����Ԫ�ص�����������
��4�������л����������̼Ԫ��+��Ԫ�ص������Ĵ�С�ж��Ƿ�����Ԫ�أ�
��5����������������ȷ��ά����C�����ʽ��Ҫȷ��ά����C�ķ���ʽ����Ҫ֪��ά����C����Է���������
��2��ʵ���������������ã�ȷ��ά����C��ĩ��ַ�Ӧ��ͬʱ�����ɵĶ�����̼��ˮ����ȫ������װ��AB��ȫ�����գ�
��3���л���ͨ���������������ȼ�����ɶ�����̼��ˮ�����������Ⱥ�ͨ��Ũ����ͼ�ʯ�ң����߷ֱ�����0.144g��0.528g����ȼ������ˮΪ0.144g��������̼Ϊ0.528g�����������غ�����ά����C��̼����Ԫ�ص�����������
��4�������л����������̼Ԫ��+��Ԫ�ص������Ĵ�С�ж��Ƿ�����Ԫ�أ�
��5����������������ȷ��ά����C�����ʽ��Ҫȷ��ά����C�ķ���ʽ����Ҫ֪��ά����C����Է���������
���
�⣺��1���ⶨά����C��̼�������������������װ��A�������յõ���ˮ�������ⶨ��Ԫ�غ���������ϴ��ƿ�е�Ũ��������ˮ������װ��ͼΪ��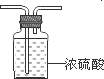 ��
��
�ʴ�Ϊ�� ��
��
��2��ʵ���������������ã�ȷ��ά����C��ĩ��ַ�Ӧ��ͬʱ�����ɵĶ�����̼��ˮ����ȫ������װ��AB��ȫ�����գ�
�ʴ�Ϊ������������������ABװ�ñ���ȫ���գ�
��3�����������Ⱥ�ͨ��Ũ����ͼ�ʯ�ң����߷ֱ�����0.144g��0.528g����ȼ������ˮΪ0.144g��������̼Ϊ0.528g��
0.528g������̼�����ʵ���Ϊ��n��CO2��=
=0.012mol������̼ԭ������Ϊ��m��C��=12g/mol��0.012mol=0.144g��̼Ԫ�ص���������Ϊ��w��C��=
��100%��40.9%��
0.144gˮ�����ʵ���Ϊ��n��H2O��=
=0.008mol��0.008molˮ�к���0.016mol Hԭ�ӣ�����Ԫ�ص�����Ϊ��1g/mol��0.016mol=0.016g����Ԫ�ص���������Ϊ��w��H��=
��100%��4.55%��
�ʴ�Ϊ��40.9%��4.55%��
��4��0.352g ά����C�к���̼Ԫ�ص�����Ϊ��0.144g��������Ԫ�ص�����Ϊ��0.016g������C��HԪ�ص�����Ϊ��0.144g+0.016g=0.160g��0.352g����ά����C��һ��������Ԫ�أ�������Ԫ�ص�����Ϊ��0.352g-0.144g-0.016g=0.192g��
��ά����C�к�����Ԫ�أ���ΪC��HԪ�ص�����֮��С��ά���ص�������
��5������C��H��O������������ȷ��ά����C�����ʽ��Ҫȷ��ά����C�ķ���ʽ����Ҫ֪��ά����C����Է���������
�������Ҫȷ��ά����C�ķ���ʽ������Ҫά����C����Է���������
 ��
���ʴ�Ϊ��
 ��
����2��ʵ���������������ã�ȷ��ά����C��ĩ��ַ�Ӧ��ͬʱ�����ɵĶ�����̼��ˮ����ȫ������װ��AB��ȫ�����գ�
�ʴ�Ϊ������������������ABװ�ñ���ȫ���գ�
��3�����������Ⱥ�ͨ��Ũ����ͼ�ʯ�ң����߷ֱ�����0.144g��0.528g����ȼ������ˮΪ0.144g��������̼Ϊ0.528g��
0.528g������̼�����ʵ���Ϊ��n��CO2��=
| 0.528g |
| 44g/mol |
| 0.144g |
| 0.352g |
0.144gˮ�����ʵ���Ϊ��n��H2O��=
| 0.144g |
| 18g/mol |
| 0.016g |
| 0.352g |
�ʴ�Ϊ��40.9%��4.55%��
��4��0.352g ά����C�к���̼Ԫ�ص�����Ϊ��0.144g��������Ԫ�ص�����Ϊ��0.016g������C��HԪ�ص�����Ϊ��0.144g+0.016g=0.160g��0.352g����ά����C��һ��������Ԫ�أ�������Ԫ�ص�����Ϊ��0.352g-0.144g-0.016g=0.192g��
��ά����C�к�����Ԫ�أ���ΪC��HԪ�ص�����֮��С��ά���ص�������
��5������C��H��O������������ȷ��ά����C�����ʽ��Ҫȷ��ά����C�ķ���ʽ����Ҫ֪��ά����C����Է���������
�������Ҫȷ��ά����C�ķ���ʽ������Ҫά����C����Է���������
���������⿼�����л������ʽ��ȷ������Ŀ�Ѷ��еȣ�ע������ȷ���л������ʽ���ṹ��ʽ�ķ������ܹ����������غ�ȷ���л���������Ƿ�����ԭ�ӣ�

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
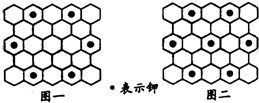 ������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������
������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������| A����������ٳ���6�ּ�ʯī������ͬ�������� | ||
| B����ij��ʯī��ԭ�ڷֲ���ͼһ��ʾ����������ʾ����C24K | ||
| C����ij��ʯī��ԭ�ӷֲ���ͼ����ʾ����������ʾ����C12K | ||
D������һ�ֻ�ɫ�ļ�ʯīC32K������K�ķֲ�Ҳ����ͼ�е����������Σ����������Kԭ��֮��ľ���Ϊʯī������4
|
�����йص縺�Ե�˵������ȷ���ǣ�������
| A������Ԫ�صĵ縺��Խ��Ԫ��ԭ�ӵĵ�һ������һ��Խ�� |
| B����Ԫ�����ڱ��У�Ԫ�ص縺�Դ�����Խ��ԽС |
| C������Ԫ�صĵ縺��һ��С�ڷǽ���Ԫ�صĵ縺�� |
| D�����γɻ�����ʱ���縺��ԽС��Ԫ��Խ���������� |
��a L���ܱ��������һ���¶��½���2A��g��+B��g��?2C��g���Ŀ��淴Ӧ�������ڿ�ʼ�������и������ʣ��ڴﵽƽ��ʱ�淴Ӧ��������һ�������ǣ�������
| A��2 mol A��1 mol B |
| B��1 mol A��1 mol B |
| C��1 mol A��2 mol B |
| D��1 mol B��1 mol C |
��һƿNa2SO3��Һ�����������ܲ��ֱ�������ijͬѧ��������ʵ�飺ȡ������Һ������Ba��NO3��2��Һ��������ɫ�������ټ�����ϡ���ᣬ��������а�ɫ�������Դ�ʵ������������ȷ���ǣ�������
| A��Na2SO3�Ѳ��ֱ����� |
| B������Ba��NO3��2��Һ�����ɵij�����һ������BaSO4 |
| C���������IJ��ܳ���һ����BaSO4��BaSO3 |
| D����ʵ�鲻��ȷ��Na2SO3�Ƿֱ����� |
�ϳɰ�����H2����ú��ˮ��Ӧ�Ƶã�����һ����ӦΪCO��g��+H2O��g��?CO2��g��+H2��g����H��0 �����CO��ת���ʿɲ��õķ����У��ټ�Сѹǿ���ڽ����¶ȣ�������ˮ������Ũ�ȣ�������CO��Ũ�ȣ���ʹ�ô�����������ȷ������ǣ�������
| A���ڢ� | B���ڢ� | C���٢� | D��ֻ�Т� |
������ڹ������ţ����ȷ���ǣ�������
| A����ķǽ����Ա�̼ǿ��ֻ���ڸ����²��ܸ������Ϸ�Ӧ |
| B�����ǹ��ɿ������ʯ����ҪԪ�أ����ڵؿ��еĺ��������е�Ԫ���оӵ�һλ |
| C����Ļ�ѧ���ʲ����ã�����Ȼ���п���������̬���� |
| D�����ڵ��ӹ�ҵ������Ҫ�İ뵼����� |