��Ŀ����
2015��11�£�һ����Ϊ���ǡ���ѧ��ɷ������Ƭ��Ϊ������������ġ�ͷ�����š���ԭ������ͨ�����������ջ��ǵ��Ӿ羵ͷ��ͼ����Ƭ�е����ǰڳ�������ȴ���ͻ�ѧ������ء���������Ϩ��ƾ��ƻ���ʹ�������ܣ������Ƕ��ÿ��Ƹ���ķ�ʽ�����������ܿ��˻�ѧʵ���е���ȷ���������������̿����������л���ʵ�������ȷ����
A. ϡ��Ũ����ʱ����ˮ�����ڻ���ע��Ũ������
B. ����ʱ��©����Һ���Һ��Ҫ������ֽ��Ե
C. ��ͷ�ιܵĹܿ�ֱ�������Թ���μ�Һ�壬�����⽦
D. ʵ����ȡ��Һ��ҩƷ��ʵ��ʱ�����û��˵��������һ��ȡ1~2mL
��ϰ��ϵ�д�
�����Ŀ
14����ʢ����������Һ���ձ��з����õ������ӵ�ͭƬ����Ƭ������������ȷ���ǣ�������
| A�� | ������������ | B�� | ����ͨ��������ͭƬ������Ƭ | ||
| C�� | �ձ���Ag+Ũ����С | D�� | ��ع���ʱ��ͭƬʧ���ӣ�����ԭ |


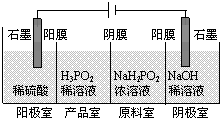 �����ᣨH3PO2����һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺
�����ᣨH3PO2����һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺ 2CH3COO����Cu2+��H2��
2CH3COO����Cu2+��H2��

 CO��+H2O�����ô�����CO��ɸ�ʵ�顣
CO��+H2O�����ô�����CO��ɸ�ʵ�顣 ��Ϊcmol/L��ô���й�ϵʽ��ȷ����( )
��Ϊcmol/L��ô���й�ϵʽ��ȷ����( )