��Ŀ����
ij��ɫ��Һ�к���K+��Cl����OH����SO ��SO
��SO ��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��

��1��ͼ���Լ��١������ʵĻ�ѧʽ�ֱ���
��________����________����________����__________����__________��
��2��ͼ������a��b��c��������������ӷֱ���a________��b________��c________��
��3����ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ_________________��
��4����ɫ��ҺC���Լ��۵���ҪĿ�� ��_____________________��
��_____________________��
��5����ɫ���� A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________��
A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________��
��6������Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��____________________��
��ϰ��ϵ�д�
�����Ŀ
9�������л�ѧ֪ʶ�����ڣ����л�ѧ֪ʶӦ�ô�����ǣ�������
| A�� | �����������ǿ�����ԣ�����ɱ�����������Գ����������� | |
| B�� | �ƹϺ��зḻ��ά����C���ȹ������ܼ���ά����C����ʧ | |
| C�� | ����Һ�м������Ƶ�Cu��OH��2����Һ��������п������������ | |
| D�� | ����ȼ�յķ���������ëΧ���ͺϳ���άΧ�� |
13��ˮ��Һ�����е��뷽��ʽ��д��ȷ���ǣ�������
| A�� | Ca��OH��2?Ca2++2OH- | B�� | NaHCO3�TNa++H++CO32- | ||
| C�� | H2SO3?2H++SO32- | D�� | BaSO4�TBa2++SO42- |
��2 mol NaOH��1 mol Ba(OH)2��2 mol Na[Al(OH)4]�Ļ����Һ������ͨ��CO2����ͨ��CO2���������ɳ��������Ĺ�ϵ����ȷ����( )
ѡ�� | A | B | C | D |
n(CO2)(mol) | 2 | 3 | 4 | 6 |
n(����)(mol) | 1 | 2 | 3 | 2 |


 ��
�� +HBr
+HBr
 b��Na2CO3��Һ c��Na2SO4��Һ
b��Na2CO3��Һ c��Na2SO4��Һ

 ��
�� ��
�� ��ע����Ӧ��������
��ע����Ӧ��������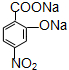 ��
��