��Ŀ����
 A��B��C��Ԫ�����ڱ���ǰ18��Ԫ���е�3�ֵ��ʣ��ס����dz����Ļ��������֮�������ͼ��ʾ��ϵ��
A��B��C��Ԫ�����ڱ���ǰ18��Ԫ���е�3�ֵ��ʣ��ס����dz����Ļ��������֮�������ͼ��ʾ��ϵ����1�����׳����³�Һ̬��A�������ǹ�̬�ǽ������ʣ����ҵĻ�ѧʽ��
��2�����׳����³���̬��A�dz����Ľ������ʣ�д��A�ͼ���һ�������·�Ӧ�Ļ�ѧ����ʽ
��3����A��B����ͬһ����ĸ�Ӳ�ȵ��ʣ�д����Ӧ�ڵĻ�ѧ��Ӧ����ʽ
���㣺������ƶ�
ר�⣺�ƶ���
��������1�����׳����³�Һ̬��ӦΪH2O��A�������ǹ�̬�ǽ������ʣ�ӦΪ̼��������CO��B��H2��C��O2����A����������̬�ǽ������ʣ�ӦΪF2������HF��B��O2��C��H2��
��2�����׳����³���̬��A�dz����Ľ������ʣ�ӦΪMg������CO2������Mg��B��C��C��O2��
��3����A��B����ͬһ����ĸ�Ӳ�ȵ��ʣ�A��C������SiO2������CO��B��Si��C��O2��
��2�����׳����³���̬��A�dz����Ľ������ʣ�ӦΪMg������CO2������Mg��B��C��C��O2��
��3����A��B����ͬһ����ĸ�Ӳ�ȵ��ʣ�A��C������SiO2������CO��B��Si��C��O2��
���
�⣺��1�����׳����³�Һ̬��ӦΪH2O��A�������ǹ�̬�ǽ������ʣ�ӦΪ̼��������CO��B��H2��C��O2����A����������̬�ǽ������ʣ�ӦΪF2������HF��B��O2��C��H2���ҵĵ���ʽΪ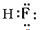 ���ʴ�Ϊ��CO��
���ʴ�Ϊ��CO��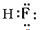 ��
��
��2�����׳����³���̬��A�dz����Ľ������ʣ�ӦΪMg������CO2������Mg��B��C��C��O2��A�ͼ���һ�������·�Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2
2MgO+C��
�ʴ�Ϊ��2Mg+CO2
2MgO+C��
��3����A��B����ͬһ����ĸ�Ӳ�ȵ��ʣ�A��C������SiO2������CO��B��Si��C��O2����Ӧ�ڵĻ�ѧ��Ӧ����ʽΪ2C+SiO2
2CO+Si��
�ʴ�Ϊ��2C+SiO2
2CO+Si��
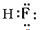 ���ʴ�Ϊ��CO��
���ʴ�Ϊ��CO��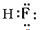 ��
����2�����׳����³���̬��A�dz����Ľ������ʣ�ӦΪMg������CO2������Mg��B��C��C��O2��A�ͼ���һ�������·�Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2
| ||
�ʴ�Ϊ��2Mg+CO2
| ||
��3����A��B����ͬһ����ĸ�Ӳ�ȵ��ʣ�A��C������SiO2������CO��B��Si��C��O2����Ӧ�ڵĻ�ѧ��Ӧ����ʽΪ2C+SiO2
| ||
�ʴ�Ϊ��2C+SiO2
| ||
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע�����������ʵ�ת����ϵ����ȷ�������ʵ�����Ϊ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ��������Ӧ���ϵ��ǣ�������
| A��Ba��NO3��2��Һ��ͨ�������SO2��3SO2+3Ba2++2NO3-+2H2O=3BaSO4+4H++2NO�� |
| B�����Ը��������Һ�еμ�H2O2��Һ��4MnO4-+4H2O2+12H+=4Mn2++7O2+10H2O |
| C���廯������Һ��ͨ����������������2Fe2++4Br-+3Cl2=3Fe3++2Br2+6Cl- |
| D��Fe2��SO4��3��Һ�м���������Na2S��Һ��2Fe3++3S2-=2FeS��+S�� |
����˵����ȷ���ǣ�������
| A����״���£�22.4L HF�к��еķ�ԭ����ĿΪNA��NA��ʾ�����ӵ������� |
| B��0.1mol/L��NH4HSO4��Һ�еμ�0.1mol/L��Ba��OH��2��Һ�������պ���ȫ��c��NH4+����c��OH-����c��SO42-����c��H+�� |
C�� 1mol��ͼ��ʾ������Ũ��ˮ��Ӧʱͨ���������Br2Ϊ4mol |
| D�������£���ӦC��s��+CO2��g��=2CO��g�������Է����У���÷�Ӧ�ġ�H��0��һ�������£�ʹ�ô����ܼӿ췴Ӧ���ʲ���߷�Ӧ���ƽ��ת���� |
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ���ǣ�������
| A����Ƭ��NaOH��Һ��Ӧ��2OH-+Al�TAlO2-+H2�� |
| B����AgCl����Һ�еμ�NaI��Һ����ɫ������ɻ�ɫ��AgCl+I-�TAgI+Cl- |
| C����KIO3����������Һ�е�KI��5I-+IO3-+3H2O�T3I2+6OH- |
| D���������������������Fe3O4+8H+�T3Fe3++4H2O |
��MgCl2��FeCl3�ı�����Һ�������ϣ��ټ�������ŨNaOH��Һ�������ķ�ӦΪ����֪��20��ʱ��Mg��OH��2��Fe��OH��3���ܽ�ȷֱ�Ϊ��9��10-4g��3��10-9g����������
| A��ֻ��Fe��OH��3�������� |
| B��Mg��OH��2��Fe��OH��3�������������� |
| C��Mg��OH��2������Fe��OH��3���� |
| D��Mg��OH��2��������Fe��OH��3���� |


 ����̬������Ϊ����ѧ��Ӧ������ͨ������ײ������ɵģ����Ǵӷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ1��1mol NO2��1mol COǡ�÷�Ӧ����CO2��NO�����е������仯ʾ��ͼ��
����̬������Ϊ����ѧ��Ӧ������ͨ������ײ������ɵģ����Ǵӷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ1��1mol NO2��1mol COǡ�÷�Ӧ����CO2��NO�����е������仯ʾ��ͼ��