��Ŀ����
16����1����AlCl3 ��Һ���ɺ������գ����õ�����Ҫ���������Al2O3���������ǣ������ӷ���ʽ��ѧ����ʽ��ʾ��Al3++3H2O?Al��OH��3+3H+��2Al��OH��3$\frac{\underline{\;����\;}}{\;}$Al2O3+3H2O��2�����ڿ��淴ӦaA��g��+bB��g��?cC��g��+dD��g����H��0��ƽ�ⳣ��K�ı���ʽΪ$\frac{{c}^{c}��C��•{c}^{d}��D��}{{c}^{a}��A��•{c}^{b}��B��}$��Kֻ���¶�Ӱ�죬200��ʱΪK1��800��ʱΪK2����K1С��K2������С��ȣ���
��3���Ȼ���ˮ������ӷ���ʽΪFe3++3H2O?Fe��OH��3+3H+�������Ȼ�����Һʱ�μ�������������������ƣ���ٽ��������ơ���ˮ�⣻�����Ȼ�����Һ�м���̼��Ʒ�ĩ������̼��������ܽ⣬��������ɫ���壬ͬʱ�к��ɫ�������ɵ�ԭ���ǣ�CaCO3������ˮ��ƽ���е�H+��Ӧ��ʹˮ��ƽ�������ƶ�������Fe��OH��3���࣬�γɺ��ɫ������
��4��һ�������µĿ��淴Ӧ2NO2 ��g���PN2O4��g����H=-92.4kJ/mol�ﵽ��ѧƽ��״̬��������������ʱ��
����������¶ȣ�ƽ���������ɫ���������dz������
�����������̶��������м���һ�����Ķ�����������ѧƽ��������Ӧ �����ƶ���
���� ��1������AlCl3����ǿ�������Σ�����Һ�д���ˮ��ƽ�⣬��ƽ���ƶ��ĽǶȷ����������⣻
��2��ƽ�ⳣ��K=$\frac{������ƽ��Ũ�ȵ���֮��}{��Ӧ��ƽ��Ũ����֮��}$��Kֻ���¶ȵ�Ӱ�죬�˷�Ӧ���ȣ������¶ȣ�ƽ�����ƣ�K����
��3���Ȼ���Ϊǿ�������Σ�ˮ������ԣ���������ǿ�������ε�ˮ�⣻�Ȼ���Ϊǿ�������Σ�ˮ������ԣ�CaCO3������ˮ��ƽ���е�H+��Ӧˮ��ƽ�����ƣ�
��4��������Ӧ���ȣ������¶�ƽ�����淴Ӧ�����ƶ���
������Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ���
��� �⣺��1���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O?Al��OH��3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���Al��OH��3���ȶ�������ʱ�ֽ�����Al2O3��2Al��OH��3$\frac{\underline{\;����\;}}{\;}$Al2O3+3H2O��
��Al2O3��Al3++3H2O?Al��OH��3+3H+��2Al��OH��3$\frac{\underline{\;����\;}}{\;}$Al2O3+3H2O��
��2��ƽ�ⳣ��K=$\frac{������ƽ��Ũ�ȵ���֮��}{��Ӧ��ƽ��Ũ����֮��}$=$\frac{{c}^{c}��C��•{c}^{d}��D��}{{c}^{a}��A��•{c}^{b}��B��}$��Kֻ���¶ȵ�Ӱ�죬�˷�Ӧ���ȣ������¶ȣ�ƽ�����ƣ�K����K1С��K2���ʴ�Ϊ��$\frac{{c}^{c}��C��•{c}^{d}��D��}{{c}^{a}��A��•{c}^{b}��B��}$���¶ȣ�С��
��3��FeCl3Ϊǿ�������Σ�Fe3+����ˮ��ʹ��Һ�����ԣ�ˮ�ⷽ��ʽΪFe3++3H2O?Fe��OH��3+3H+��FeCl3Ϊǿ�������Σ�ˮ�������ԣ�����������������ˮ�⣻
�Ȼ���ˮ�������Fe3++3H2O?Fe��OH��3+3H+��CaCO3������ˮ��ƽ���е�H+��Ӧ����Ӧ����H+��ʹc��H+����С������ˮ��ƽ�������ƶ�������Fe��OH��3���࣬�γɺ��ɫ������
�ʴ�Ϊ��CaCO3+2H+=Ca2++H2O+CO2���� H+���ң�
��4��������ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ�������ɫ����ʴ�Ϊ�����
�����������̶������м���һ�����Ķ�������������Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ����ʴ�Ϊ������Ӧ��
���� ���⿼��ƽ���ƶ���Ӱ�����أ���Ŀ�ѶȲ�����ע�����ƽ���ƶ�ԭ����

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�| A�� | 224 mL | B�� | 168 mL | C�� | 112 mL | D�� | 448 mL |
| A�� | Al2O3 | B�� | MgO | C�� | NaHCO3 | D�� | Al��OH��3 |
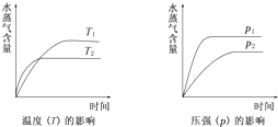
| A�� | CH3CH2OH?CH2=CH2��g��+H2O��g������H��0 | |
| B�� | CO2��g��+H2��g��?CO��g��+H2O��g������H��0 | |
| C�� | CO2��g��+2NH3�� g��?CO��NH2��2��s��+H2O��g������H��0 | |
| D�� | 2C6H5CH2CH3��g��+O2��g��?2C6H-5CH=CH2��g��+2H2O��g������H��0 |
| A�� | ͭ��Ũ����Ϊԭ����������ͭ | B�� | �����������Ʊ�һ�ȼ��� | ||
| C�� | ��ϩ��HCl��ȡ������ | D�� | �ɷ�Ӧ2SO2+02?2SO3��SO3 |
| A�� |  | B�� |  | C�� |  | D�� |  |
 ���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������SO2���ŷ�������8%���������NOx���ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������SO2���ŷ�������8%���������NOx���ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����