��Ŀ����
7��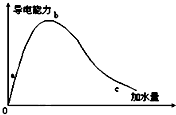 ��һ���¶��£��������ˮϡ�͵Ĺ����У���Һ�ĵ���������ͼ��ʾ����ش�
��һ���¶��£��������ˮϡ�͵Ĺ����У���Һ�ĵ���������ͼ��ʾ����ش���1����0���㵼������Ϊ0�����ɴ���Ϊ���ۻ�����ڹ̡�Һ̬ʱ�����������ƶ������ӣ����Բ����磮
��2��a��b��c������Һ��pHֵ��С�����˳��Ϊb��a��c��
��3��a��b��c�����е���������ǣ�c��
��4����ʹc����Һ��[CH3COO-]������Һ��pHֵҲ���ɲ�ȡ�Ĵ�ʩΪ��
�ټ�NaOH��s�����ڼ�Na2CO3��s�����ۼ�����ý�������п��þ�ȣ���
���� ��1����Һ��ͨ�����ӵĶ����ƶ��γɵ����ģ�
��2����Һ�ĵ�������������Ũ�ȳ����ȣ�
��3����ҺԽϡ������ĵ���̶�Խ��
��4����ʹC����Һ��[CH3COO-]����[H+]��С����������Һ�м���ijЩ�������ӷ�Ӧ�����ʣ�
��� �⣺��1����Һ��ͨ�����ӵĶ����ƶ��γɵ����ģ��������д����Է��Ӵ��ڣ����������ӣ����Ա�������磬
�ʴ�Ϊ������Ϊ���ۻ�����ڹ̡�Һ̬ʱ�����������ƶ������ӣ����Բ����磻
��2����Һ�ĵ�������������Ũ�ȳ����ȣ�����ͼ��֪����Һ����������С˳����b��a��c����������Ũ����С����˳����c��a��b����pH��С�����˳��Ϊb��a��c��
�ʴ�Ϊ��b��a��c��
��3����ҺԽϡ������ĵ���̶�Խ����ͼ��֪����Һ�����С˳����c��b��a�����Դ������̶�������c��
�ʴ�Ϊ��c��
��4����ʹC����Һ��[CH3COO-]����[H+]��С����������Һ�м���ijЩ�������ӷ�Ӧ�����ʣ�������С�ڴ�����Ρ����ý����ȣ�
�ʴ�Ϊ���ټ�NaOH��s�����ڼ�Na2CO3��s�����ۼ�����ý�������п��þ�ȣ���
���� ���⿼����������ʵĵ��룬��ȷ��Һ����������Ӱ�����ء�������ʵ���̶�����ҺŨ�ȵĹ�ϵ�ǽⱾ��ؼ�����ȷ����ͼ�ɣ���Ŀ�ѶȲ���

| A�� | 1molNa2O2��SO2��ȫ��Ӧʱת�Ƶ�����ΪNA | |
| B�� | 18g��ˮ��D2O�������ĵ�����Ϊ10NA | |
| C�� | 0.5molNH4HSO4�����У�����H+��ĿԼΪ0.5 NA | |
| D�� | ���³�ѹ�£�3gHCHO��CH3COOH�Ļ�����к���0.4NA��ԭ�� |
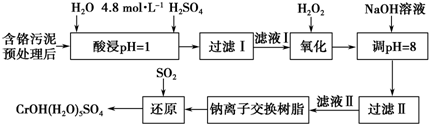
���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��1��ʵ������98%���ܶ���1.84g/cm3����Ũ��������250mL 4.8mol•L-1��H2SO4��Һ�����õIJ����������ձ�������������Ͳ�⣬����250mL����ƿ����ͷ�ιܣ���Ҫȡ��65.2ml98%���ܶ���1.84g/cm3����Ũ���ᣮ
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�����߷�Ӧ�¶ȣ������������ı������������㣩��
��3��H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��2Cr3++3H2O2+H2O=Cr2O72-+8H+��
��4�������£�����������������������ʽ����ʱ��Һ��pH�����
| ������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | - | - | - |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9����9�ܽ⣩ |
��5�������ӽ�����֬�ķ�Ӧԭ��ΪMn++nNaR��MRn+nNa+�����������ӽ�����֬��ȥ����Һ���еĽ�����������Ca2+��Mg2+��
��6��д��������������SO2���л�ԭʱ������Ӧ�Ļ�ѧ����ʽ��3SO2+2Na2CrO4+12H2O=2CrOH��H2O��5SO4��+Na2SO4+2NaOH��
��CH4��g��+4NO2 ��g���T4NO��g��+CO2 ��g��+2H2 O��g����H1=-574kJ•mol-1
��CH4 ��g��+4NO��g���T2N2 ��g��+CO2 ��g��+2H2 O��g����H2=-1160kJ•mol-1
����ѡ���ȷ���ǣ�������
| A�� | CH4 ��g��+2NO2 ��g���TN2 ��g��+CO2 ��g��+2H2 O��g����H=-867 kJ•mol-1 | |
| B�� | CH4 ��g��+4NO2 ��g���T4NO��g��+CO2 ��g��+2H2O��l����H3����H1 | |
| C�� | ����0.2 mol CH4��ԭNO2��N2����Ӧ�зų�������һ��Ϊ173.4 kJ | |
| D�� | ���ñ�״����2.24 L CH4��ԭNO2��N2������������ת�Ƶĵ���Ϊ0.8mol |

| A�� | a��b��c����������Ӧ��ˮ���������ǿ����ϵ��c��b��a | |
| B�� | a��b��c���⻯��ˮ��Һ������ǿ����ϵ��a��b��c | |
| C�� | a��d��ԭ�ӽ�Ͽ����γɴ�3����λ����ɵ������� | |
| D�� | ԭ�Ӱ뾶�ɴ�С��˳����c��b��d��a |
| A�� | ����Ӧ��Ũ�� | B�� | �����¶� | C�� | ����ѹǿ |
| A�� | ��ӦMnO2+ZnS+2H2SO4�TMnSO4+ZnSO4+S+2H2O��1molMnO2������ת��2mol���� | |
| B�� | ������ˮ���Σ�CaCO3��BaSO4�ȣ������������ | |
| C�� | NaHSO4������״̬�µĵ��뷽��ʽΪ��NaHSO4�TNa++HSO4- | |
| D�� | ��Ҫͨ��ſɽ��е��У���⡢��Ӿ�����롢��ơ��绯��ʴ |
 2N02 ��H<0��N0��ƽ��ת�������¶ȵĹ�ϵ��ͼ�б���a��b��c��d�ĵ㣬���б�ʾδ�ﵽƽ��״̬����v(��)��v(��)�ĵ��� ( )
2N02 ��H<0��N0��ƽ��ת�������¶ȵĹ�ϵ��ͼ�б���a��b��c��d�ĵ㣬���б�ʾδ�ﵽƽ��״̬����v(��)��v(��)�ĵ��� ( )
 ʵ���ҳ������·�����ȡ������MnO2+4HCl��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��������һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ũҵ������������������Ҫ��Ӧ�ã����������ѧ֪ʶ�ش��������⣺
ʵ���ҳ������·�����ȡ������MnO2+4HCl��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��������һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ũҵ������������������Ҫ��Ӧ�ã����������ѧ֪ʶ�ش��������⣺