��Ŀ����
14���±���Ԫ�����ڱ���һ���֣�������ĸ�ֱ����һ��Ԫ�أ�
��1��mԪ�������ڱ��е�λ���ǵ������ڢ�A��
��2�������й�˵����ȷ����ABCF������ĸ����
A��b��c��dԪ�صķǽ�����������
B��f��g��hԪ�ص�ԭ�Ӱ뾶��С
C��md2��bd2�Ļ�ѧ�������ƣ�������������
D��e��n����ۺ����������ǿ����e��n
E��a��f�ֱ���d��ɵĻ�������������ѧ��������ȫ��ͬ
F���ñ���ֻ��5��Ԫ����ɵĵ��ʾ��е�����
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����ĵ���ʽ��
 �����к��е�
�����к��е���ѧ������Ϊ���ۼ������Ӽ���
���� ��Ԫ�������ڱ��е�λ�ã���֪aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCa��
��1����m��λ�ÿ�֪��λ�����ڱ��е������ڢ�A�壻
��2��A��ͬ�����������Ԫ�طǽ�������ǿ��
B��ͬ�����������ԭ�Ӱ뾶��С��
C��SO2��CO2��Ϊ��������������������ԣ�
D��FԪ��û����ۺ����
E��a��d��ɵĻ�������H2O��H2O2�������ڹ��ۻ����f�ֱ���d��ɵĻ�������Na2O��Na2O2���������ӻ����
F���������ʵ��磬ʯīҲ���磻
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����NH4Cl����笠������������ӹ��ɣ�
��� �⣺��Ԫ�������ڱ��е�λ�ã���֪aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCa��
��1����m��λ�ÿ�֪��λ�����ڱ��е������ڢ�A�壬�ʴ�Ϊ���������ڢ�A�壻
��2��A��ͬ�����������Ԫ�طǽ�������ǿ��b��c��dԪ�صķǽ���������ǿ����A��ȷ��
B��ͬ�����������ԭ�Ӱ뾶��С��f��g��hԪ�ص�ԭ�Ӱ뾶��С����B��ȷ��
C��SO2��CO2��Ϊ�����������ѧ�������ƣ�SԪ�ش����м��̬��CԪ�ش�����ۣ������������ԣ���C��ȷ��
D��FԪ��û����ۺ����ᣬ��D����
E��a��d��ɵĻ�������H2O��H2O2�������ڹ��ۻ�������й��ۼ���f�ֱ���d��ɵĻ�������Na2O��Na2O2���������ӻ�����������Ӽ����������ƻ����й��ۼ�����E����
F����������Na��Mg��Al��Ca���Ե��磬ʯīҲ���磬��F��ȷ��
��ѡ��ABCF��
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����NH4Cl����笠������������ӹ��ɣ�����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ѶȲ���ע������ڱ���Ԫ�������ɵ��������գ���2����Dѡ��Ϊ�״��㣬ѧ��������FԪ��û����ۺ����ᣮ

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д� ��NaOH��Һ����SO2�������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ��������������Ĥ���ѭ������������ͼ��������˵������ȷ���ǣ�������
��NaOH��Һ����SO2�������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ��������������Ĥ���ѭ������������ͼ��������˵������ȷ���ǣ�������| A�� | ������������ǿ | |
| B�� | diluent��concentrated�����ĺ���ΪŨ���ġ�ϡ�͵� | |
| C�� | ���������ӵ��������ӽ���Ĥ | |
| D�� | �ù����еĸ���Ʒ��ҪΪH2SO4 |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
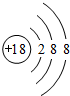 ��
����2��д���ݺ͢�����Ԫ�ص�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽAl��OH��3+KOH=KAlO2+2H2O��
��3���õ���ʽ��ʾ��Ԫ�����Ԫ���γɻ�����Ĺ���
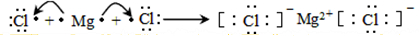 ��
����4���١��ڡ��ޡ�������Ԫ�ص�����������ˮ������������ǿ����HClO4���ѧʽ����
��5���١�����Ԫ���γɵĻ��������ʽ��
 ���û���������ں��м��Լ�������ԡ��Ǽ��ԡ�����
���û���������ں��м��Լ�������ԡ��Ǽ��ԡ�������6������֤���ߵĵ��ʱȢ�ĵ���������ǿ�����ӷ�Ӧ����ʽΪ2Br-+Cl2=Br2+2Cl-��
| ���� | ��Na2CO3 | ��NaHCO3 | ��Na2SiO3 | ��Na2SO3 | ��NaHSO3 | ��NaClO |
| pH | ��11.6 | 9.7�� | ��12.3 | ��10.0 | ��4.0 | ��10.3 |
��1�������£���ͬ���ʵ���Ũ�ȵ�����ϡ��Һ����������ǿ������˳����B��C��D��A����A��B��C��D��ʾ����
A��H2SiO3 B��H2SO3 C��H2CO3 D��HClO
��2����Ҫ������ˮ�д������Ũ�ȣ�������ˮ�м����ϱ��е�������NaHCO3��NaClO���ѧʽ����
��3����д������NaClO��Һ��ͨ������CO2�Ļ�ѧ����ʽ��NaClO+CO2+H2O=HClO+NaHCO3��
��4����Ũ�ȵ�H2SO3��NaHSO3���Һ������������ǿ���ǿ����Һ��pHֵ��û�����Ա仯�������ԭ�Ӽ�ʱ������Ӧ��H2SO3+OH-=HSO3-+H2O������ʱ������Ӧ��HSO3-+H+=H2SO3�������ӷ���ʽ��ʾ����
�ٻ�������ѹǿ
�ڻ��������ܶ�
�۸��������ʵ����ʵ���Ũ��
������������ʵ���
�ݻ�������ƽ����Է�������
��A ��������
| A�� | �٢ڢۢ� | B�� | �ڢۢݢ� | C�� | �ڢۢܢ� | D�� | �٢ۢݢ� |
| A�� | ��������ϡ���ᷴӦ��Fe2O3+6H+=2Fe2++3H2O | |
| B�� | ̼��������Һ�м�ϡ���CO32-+2H+=H2O+CO2�� | |
| C�� | ����������Һ����ϡ�����У�OH-+H+ H2O | |
| D�� | Cu��OH��2����H2SO4��2H++Cu��OH��2=Cu2++2H2O |
| A�� | S+O2�T2SO2����H=-269kJ/mol����Ӧ�ȣ� | |
| B�� | 2NO2��g���TO2��g��+2NO��g������H=+116.2kJ/mol����Ӧ�ȣ� | |
| C�� | C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g������H=-1367.0kJ/mol��ȼ���ȣ� | |
| D�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=+57.3kJ/mol���к��ȣ� |