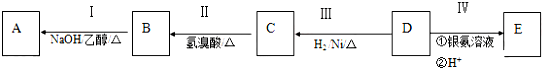
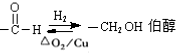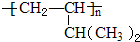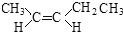��Ŀ����
16��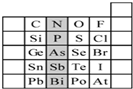 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�����Ԫ�ط��Ż�ѧʽ��գ�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�����Ԫ�ط��Ż�ѧʽ��գ���1����ʾԪ����
�ٷǽ�������ǿ��Ԫ���ǣ�F
�ڽ�������ǿ��Ԫ���ǣ�Pb
��ԭ�Ӱ뾶��С���ǣ�F
����ۺ�����������ǿ����HClO4
�ݾ��������뵼����ϵ�Ԫ���ǣ�Si��ֻдһ�֣�
����Ӱ��������Ԫ�����ڱ��е�VA�壬����Ԫ�ص�������۾�Ϊ+5
��2������Ԫ�������ɣ��Ƶ���
������ǿ����H3AsO4��H3PO4���á�����������ʾ����ͬ����
���ȶ��ԣ�H2S��HCl
�۷е㣺HF��HCl
�ܻ�ԭ�ԣ�I-��Br-
����O��F��S��Cl����Ԫ���У��ǽ�������ӽ����ǣ�D
A��O��F B��F��S C��S��Cl D��O��Cl
��3������ͬ����Ԫ�����ʵ������Ժ͵ݱ��Խ���Ԥ�⣺
�ٹ���Se��Ԥ����ȷ���ǣ�BC
A��������Se���������� B��Se����������ˮ�����ܺ�NaOH������Ӧ
C���⻯��Ļ�ѧʽΪH2Se D��������������ֻ��SeO3
����֪Cl2��ˮ��Һ���ܺ�SO2��Ӧ��Cl2+2H2O+SO2�TH2SO4+2HCl��д��Br2��ˮ��Һ�к�SO2��Ӧ�����ӷ�Ӧ����ʽBr2+2H2O+SO2�T4H++SO42-+Cl-��
���� ��1����ͬ�����������Ԫ�صķǽ�������ǿ��ͬ�������϶���Ԫ�صķǽ����Լ�����
��ͬ�����������Ԫ�صĽ����Լ�����ͬ�������϶���Ԫ�صĽ�������ǿ��
��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
����ۺ�����������ǿ���Ǹ����
���ڽ����ͷǽ����ֽ��ߴ���Ԫ�ص��������뵼�壻
������Ԫ���У�ԭ����������������������������ϼ�����ȣ�
��2����Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��
��Ԫ�صĽ�����Խǿ�����⻯��Խ�ȶ���
�ۺ�����������ʷе�ϸߣ�
�ܷǽ�����Խǿ�������ӻ�ԭ��Խ����
�ݵ縺�������Ԫ�أ���ǽ����������
��3���ٸ���ͬһ����Ԫ�����ʵ������Ժ͵ݱ��Խ��
����Ͷ�������Ӧ������ԭ��Ӧ��������������ᣮ
��� �⣺��1����ͬ�����������Ԫ�صķǽ�������ǿ��ͬ�������϶���Ԫ�صķǽ����Լ������ǽ�������ǿ��Ԫ����Ԫ�����ڱ����Ͻǣ�ΪFԪ�أ��ʴ�Ϊ��F��
��ͬ�����������Ԫ�صĽ����Լ�����ͬ�������϶���Ԫ�صĽ�������ǿ����������ǿ��Ԫ����Ԫ�����ڱ����½ǣ�ΪPbԪ�أ��ʴ�Ϊ��Pb��
��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��С��Ԫ����Ԫ�����ڱ����Ͻǣ�ΪFԪ�أ��ʴ�Ϊ��F��
��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��F��OԪ��û����ۺ����ᣬ������ۺ�����������ǿ����HClO4���ʴ�Ϊ��HClO4��
���ڽ����ͷǽ����ֽ��ߴ���Ԫ�ص��������뵼�壬��Si���ʴ�Ϊ��Si��
������Ԫ���У�ԭ����������������������������ϼ�����ȣ��⼸��Ԫ��ԭ�ӵ�������������5�������⼸��Ԫ��λ�ڵ�VA�壬�γɵ�����ϼ�Ϊ+5���ʴ�Ϊ��VA��+5��
��2����Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��PԪ�صķǽ����Դ���AsԪ�أ���������ǿ����H3AsO4��H3PO4���ʴ�Ϊ������
��Ԫ�صĽ�����Խǿ�����⻯��Խ�ȶ���S�ķǽ�����С��ClԪ�أ������ȶ��ԣ�H2S��HCl���ʴ�Ϊ������
��ͬһ�����У�������������ʷе����������Ԫ���⻯��ķе㣬HF�к��������HCl��û����������Էе�HF��HCl���ʴ�Ϊ������
��ͬһ�����У������ӵĻ�ԭ������ԭ���������������ǿ�����Ի�ԭ��I-��Br-���ʴ�Ϊ������
�ݵ縺�������Ԫ�أ���ǽ����������OԪ�غ�ClԪ�صĵ縺�Խӽ���������ǽ����Խӽ�����ѡ��D��
��3����A����Ϊ���壬���Գ�����Se�����ǹ��壬��A����
B��Se����������ˮ����Ϊ�ᣬ�ܺ�NaOH�����кͷ�Ӧ����B��ȷ��
C��ͬһ�����У����⻯��Ļ�ѧʽ��ͬ������Se�⻯��Ļ�ѧʽΪH2Se����C��ȷ��
D���������������֪����������������SeO3��SeO2����D����
��ѡ��BC��
�������ǿ�����ԣ��ܽ�������������Ϊ���ᣬͬʱ��������ԭΪ�����ᣬ���ӷ�Ӧ����ʽΪBr2+2H2O+SO2�T4H++SO42-+Cl-��
�ʴ�Ϊ��Br2+2H2O+SO2�T4H++SO42-+Cl-��
���� ���⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���������Ԫ���������ǽⱾ��ؼ���ע��Ի���֪ʶ���������գ�

| A�� | Ԫ��X �ļ���̬�⻯������ȶ��Ա�W ��ǿ | |
| B�� | Ԫ��W ������������Ӧˮ��������Ա�Z ���� | |
| C�� | ������YX��ZX2��WX3 �л�ѧ����������ͬ | |
| D�� | Ԫ��X �ļ���̬�⻯��ķе��W�ĵ� |
| A�� | 4mL | B�� | 10.8mL | C�� | 1.2mL��4mL | D�� | 8mL��10.8mL |
| A�� | �ڹ��ۻ�������һ�����й��ۼ� | |
| B�� | �ɷǽ���Ԫ����ɵĻ�����һ���ǹ��ۻ����� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | ˫ԭ�ӵ��ʷ����еĹ��ۼ�һ���ǷǼ��Լ� |
��1������������ȷ����AD��������ĸ��
A��CH2O��ˮ���Ӽ����γ����
B��CH2O��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�����к���6���Ҽ���1����м���C6H6�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
��2��Mn��Fe�IJ��ֵ������������±���
| Ԫ �� | Mn | Fe | |
| ������ /kJ•mol-1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
��3������Ԫ��ԭ�ӵ���Χ�����Ų����ɽ�Ԫ�����ڱ�����������Ti����d����Ti��һ��������X���侧���ṹ��ͼ1��ʾ����X�Ļ�ѧʽΪTiO2��
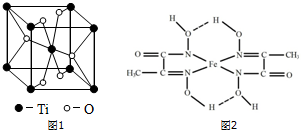
��4��ij���Ļ�����ṹ��ʽ��ͼ2��ʾ
����������������и��ǽ���Ԫ�ص縺���ɴ�С��˳��ΪO��N��C��H ����Ԫ�ط��ű�ʾ��
����ͼ2���á���������������ӵ���λ����
��5��NiO��������������Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2-����λ��Ϊ6���⼸��O2-���ɵĿռ乹��Ϊ�������壮��֪Ni2+��O2-�ĺ˼��Ϊanm��NiO��Ħ������ΪM g/mol�������ӵ�������NA��ʾ����þ�����ܶ�Ϊ$\frac{M��1{0}^{21}}{2{a}^{3}{N}_{A}}$ g/cm3��

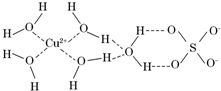 �������ڵ�����������γ�������ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£�
�������ڵ�����������γ�������ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£�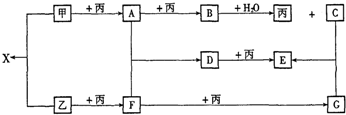
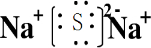 ��GSO3��
��GSO3��