��Ŀ����
3��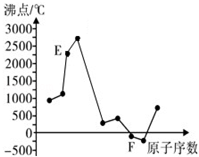 A��B��C��D��E��F��Ԫ�����ڱ���ǰ20��Ԫ�أ����ǵ�ԭ��������������A��B�ɷֱ���C��ɳ���������AC��AC2��BC��BC2��DԪ�ص���ɫ��Ӧ�ʻ�ɫ��E��F��Ԫ�ص��ʵķе���Ԫ��ԭ�������Ĺ�ϵ��ͼ��ͼ��ԭ��������������
A��B��C��D��E��F��Ԫ�����ڱ���ǰ20��Ԫ�أ����ǵ�ԭ��������������A��B�ɷֱ���C��ɳ���������AC��AC2��BC��BC2��DԪ�ص���ɫ��Ӧ�ʻ�ɫ��E��F��Ԫ�ص��ʵķе���Ԫ��ԭ�������Ĺ�ϵ��ͼ��ͼ��ԭ����������������1��A��Ԫ�����ڱ��е�λ���ǵڶ����ڢ�A�壮
��2��B2�ĵ���ʽΪ
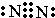 ��������AC2�ĽṹʽO=C=O��C��D��F��Ӧ�ļ����Ӱ뾶��С�����˳��ΪNa+��O2-��Cl-�������ӷ��ű�ʾ����
��������AC2�ĽṹʽO=C=O��C��D��F��Ӧ�ļ����Ӱ뾶��С�����˳��ΪNa+��O2-��Cl-�������ӷ��ű�ʾ������3��C��F�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ������������O3��Cl2��ClO2�ȣ�д�������������ʵĻ�ѧʽ����
��4��������Y��C��E��Ԫ����ɣ���Y��B������A���ʰ�1��1��3��һ�������·�Ӧ�ɵõ�Z��AC��Z�Ļ�ѧʽΪAlN��
��5��D�����������Ӧˮ���������E������������Ӧˮ�������Ӧ�������ӷ���ʽΪ��OH-+Al��OH��3�TAlO2-+H2O��
���� A��B��C��D��E��F��Ԫ�����ڱ���ǰ20��Ԫ�أ����ǵ�ԭ��������������DԪ�ص���ɫ��Ӧ�ʻ�ɫ����DΪNa��A��B�ɷֱ���C��ɳ���������AC��AC2��BC��BC2��Ӧ��C��N��OԪ���γɵĻ������AΪ̼Ԫ�ء�BΪNԪ�ء�CΪOԪ�أ���ͼ��֪FΪ���壬���ԭ��������֪��E��F���ڵ������ڣ����ʷе���ߣ�ӦΪԭ�Ӿ���Si����EΪAl��FΪCl���ݴ˽��
��� �⣺A��B��C��D��E��F��Ԫ�����ڱ���ǰ20��Ԫ�أ����ǵ�ԭ��������������DԪ�ص���ɫ��Ӧ�ʻ�ɫ����DΪNa��A��B�ɷֱ���C��ɳ���������AC��AC2��BC��BC2��Ӧ��C��N��OԪ���γɵĻ������AΪ̼Ԫ�ء�BΪNԪ�ء�CΪOԪ�أ���ͼ��֪FΪ���壬���ԭ��������֪��E��F���ڵ������ڣ����ʷе���ߣ�ӦΪԭ�Ӿ���Si����EΪAl��FΪCl��
��1��AΪ̼Ԫ�أ���2�����Ӳ㣬����������Ϊ4������Ԫ�����ڱ��еڶ����ڢ�A�壬�ʴ�Ϊ���ڶ����ڢ�A�壻
��2��N2������Nԭ��֮���γ�3�Թ��õ��Ӷԣ������ʽΪ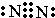 ��������CO2�ĽṹʽΪO=C=O�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��С�����˳��ΪNa+��O2-��Cl-��
��������CO2�ĽṹʽΪO=C=O�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��С�����˳��ΪNa+��O2-��Cl-��
�ʴ�Ϊ��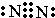 ��O=C=O��Na+��O2-��Cl-��
��O=C=O��Na+��O2-��Cl-��
��3��O��Cl�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ���������У�O3��Cl2��ClO2�ȣ��ʴ�Ϊ��O3��Cl2�ȣ�
��4��������Y��O��Al��Ԫ�����ΪAl2O3����Al2O3��N2��C��1��1��3��һ�������·�Ӧ�ɵõ�Z��CO��ӦΪAl2O3+N2+3C�TZ+3CO����ԭ���غ��֪��ZΪAlN��
�ʴ�Ϊ��AlN��
��5��D�����������Ӧˮ����ΪNaOH��E������������Ӧˮ����ΪAl��OH��3�����߷�Ӧ���ӷ���ʽΪ����
�ʴ�Ϊ��OH-+Al��OH��3�TAlO2-+H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã�ע�����������ԭ�Ӿ���Si�е���ߣ���������Ԫ�ػ�����֪ʶ��ע�����֪ʶ��ȫ�����գ�

| A�� | ��Һ��OH-Ũ��Ϊ0.03mol•L-1 | |
| B�� | ����Һ�к�Na+����Ϊ0.015NA | |
| C�� | ��ԭ��Һ�м���470mL����ˮ���� | |
| D�� | ����Һ�к�����ԭ�Ӹ�������0.015NA |
 ��ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã���Ԫ�ط��Ż�ѧ����ش��������⣺
��ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã���Ԫ�ط��Ż�ѧ����ش��������⣺| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
A��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2��
B��SiO2+2C$\frac{\underline{\;����\;}}{\;}$ Si+2CO��
C��Na2SiO3+CO2+2H2O�TH2SiO3��+Na2CO3
D��CH4��SiH4�ȶ�
��2���١��ܡ�������Ԫ����ɻ�����������ѧ�����������Ӽ������ۼ���
��3���ܡ��ݡ������γɵļ����Ӱ뾶�ɴ�С��˳��O2-��Na+��Al3+������Ԫ�ط��ű�ʾ��
��4���١��ڡ�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ11��5��1��ɵ��л��������к�������-CH3��ͬ���칹����4�֣�
��5��ijͬѧ���ʵ��֤���ڡ��ۡ��ߵķǽ�����ǿ����ϵ��
����Һa��b�ֱ�Ϊ���ᣬ����NaHCO3��Һ��
����Һc�е����ӷ���ʽΪSiO32-+CO2+H2O=H2SiO3��+CO32-��SiO32-+2CO2+2H2O=H2SiO3��+2HCO3-��
| A�� | 1molCH3+����̼�����ӣ����еĵ�����ĿΪ9NA | |
| B�� | 27g Al�ڱ�״���µ�22.4L Cl2��ȼ�գ�ת�Ƶĵ�������Ϊ3 NA | |
| C�� | 0.2 mol•L-1��Na2CO3��Һ�к���CO32-����Ŀһ��С��0.2NA | |
| D�� | 7.8gNa2S��Na2O2�Ļ�����к��е�����������Ϊ0.1 NA |
| A�� | ���� | B�� | �ɺ� | C�� | ������ն� | D�� | ����ЧӦ |
| A�� | NaCl | B�� | NaCl��NaBr��NaI | C�� | NaBr��NaI | D�� | NaI |
| A�� | Na2SO4��Һ | B�� | FeCl3��Һ | C�� | Cu��NO3��2��Һ | D�� | ϡ���� |
 ԭ���������������A��B��C��D��E��F����Ԫ�أ�����A�Ļ�̬ԭ����3����ͬ�ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��F��Cλ��ͬһ���壬F���ڵ�һ�������ڣ�
ԭ���������������A��B��C��D��E��F����Ԫ�أ�����A�Ļ�̬ԭ����3����ͬ�ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��F��Cλ��ͬһ���壬F���ڵ�һ�������ڣ�