��Ŀ����
12�� A�ǻ�ѧʵ������������л����������ˮ����������ζ�����ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ��
A�ǻ�ѧʵ������������л����������ˮ����������ζ�����ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ����1��д��A�Ļ�ѧʽC2H6O
��2�����з�Ӧ�Ļ�ѧ����ʽ
��Ӧ�٣�2CH3CH2OH+2Na��2CH3CH2ONa+H2��
��Ӧ�ڣ�CH3CH2OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O
��3���ȽϷ�Ӧ�����ƺ�ˮ��Ӧ��������ʲô��ͬ�Ͳ�ͬ��
��ͬ�㣺������ɫ���ݲ��������������ų�
��ͬ�㣺�Ҵ����Ʒ�Ӧʱ���Ƴ���Һ���£���ӦҲ��ƽ�ȣ�
���� A�ǻ�ѧʵ������������л����������ˮ����������ζ��������ȵ�ͭ˿��Ӧ�õ�D������Na��Ӧ���������ᷴӦ��A�����ǻ�����AΪCH3CH2OH����CΪCH3COOCH2CH3��DΪCH3CHO��CH3CH2OHȼ�����ɶ�����̼��ˮ��CH3CH2OH��Na��Ӧ����CH3CH2ONa���������ݴ˽��
��� �⣺A�ǻ�ѧʵ������������л����������ˮ����������ζ��������ȵ�ͭ˿��Ӧ�õ�D������Na��Ӧ���������ᷴӦ��A�����ǻ�����AΪCH3CH2OH����CΪCH3COOCH2CH3��DΪCH3CHO��CH3CH2OHȼ�����ɶ�����̼��ˮ��CH3CH2OH��Na��Ӧ����CH3CH2ONa��������
��1��AΪ�Ҵ�����ѧʽΪC2H6O���ʴ�Ϊ��C2H6O��
��2����Ӧ��Ϊ2CH3CH2OH+2Na��2CH3CH2ONa+H2����
��Ӧ��ΪCH3CH2OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O��
�ʴ�Ϊ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����CH3CH2OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O��
��3�������Ҵ����ƺ�ˮ��Ӧ�ж�����ɫ���ݲ��������������ų����Ƶ��ܶȱ��Ҵ����Ƴ���Һ���£��Ҵ����ǻ��ⲻ��ˮ�еĻ��ã���������ˮ�ķ�Ӧ���ң���ӦҲ��ƽ�ȣ�
�ʴ�Ϊ��������ɫ���ݲ��������������ų����Ҵ����Ʒ�Ӧʱ���Ƴ���Һ���£���ӦҲ��ƽ�ȣ�
���� ���⿼���л�����ƶϣ��Ƚϻ������漰����ȩ�����ᡢ����������ת����ע�����֪ʶ�����գ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
��֪��i��CR-39����ṹ��ʽ�ǣ�

ii�����봼�����·�Ӧ��
RCOOR��+R��OH$��_{��}^{����}$RCOOR��+R��OH��R��R�䡢R�����������
��1����ϩת��ΪA�ķ�Ӧ�����Ǽӳɷ�Ӧ��
��2����D��E�ķ����У���ֻ��һ�ֻ�ѧ��������ԭ�ӣ�
��D�Ľṹ��ʽ��
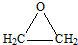 ��
����EΪ��Ԫ��״�����E��CH3OH��Ӧ�Ļ�ѧ����ʽ��
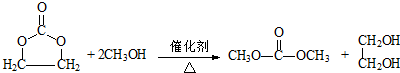 ��
����3��G������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
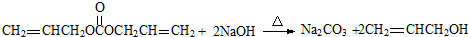 ��
����4��F��һ��ͬ���칹��K��������в�ͬ��ѧ��������ԭ�Ӹ�������3��1��1��1��������NaHCO3��Ӧ��
��K�ܷ�����ȥ��Ӧ�����ɵ��л���Ľṹ��ʽ��
 ��
����K��һ�������ºϳɸ߷��ӻ�����Ļ�ѧ����ʽ��
 ��
����5�������й�C��������ȷ���ǣ���д��ţ�acd��
| a���������ᷢ��������Ӧ | b�������Ҵ�����������Ӧ |
| c��1mol C�������2mol Na��Ӧ | d��C��ͬ���칹�岻�ܷ���������Ӧ |
| A�� | CH3CH��OH��COOH | B�� | HO��CH2��2CHO | C�� | HOOC-COOH | D�� | CH3CH2COOH |
| A�� |  �����ƣ�2-���Ҵ� �����ƣ�2-���Ҵ� | B�� | �۱�ϩ�����ڣ� | ||
| C�� | ��������Ľṹ��ʽ��C2H4O2 | D�� | ����ģ��Ϊ �ķ��ӿɷ����ӳɷ�Ӧ �ķ��ӿɷ����ӳɷ�Ӧ |
| A�� | ���� | B�� | ��Ƭ | C�� | KSCN��Һ | D�� | ʯ����Һ |
| A�� | �� | B�� | С | ||
| C�� | ��ͬ | D�� | ���ܱ��Ҳ���ܱ�С |
 ������Ȼ������ڹ����У���һ�ֳ��õ�ֲ���ζ�ͣ���ҵ����Ҫ�ǰ�����·�ߺϳɵģ�
������Ȼ������ڹ����У���һ�ֳ��õ�ֲ���ζ�ͣ���ҵ����Ҫ�ǰ�����·�ߺϳɵģ�

 ���������й����ŵ��Լ�Ϊ������Һ�����Ƶ�Cu��OH��2����Һ��
���������й����ŵ��Լ�Ϊ������Һ�����Ƶ�Cu��OH��2����Һ�� ��
�� ��
��

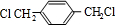 ��B
��B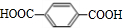 ��CHOCH2CH2OH��
��CHOCH2CH2OH�� ��
�� ʵ���ҷ���һƿ��ǩ�������Һ����ͼ��ʾ��ͬѧ���룬����Һ������C��������ĸ��ţ�
ʵ���ҷ���һƿ��ǩ�������Һ����ͼ��ʾ��ͬѧ���룬����Һ������C��������ĸ��ţ�