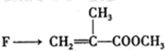��Ŀ����
ij������ܺ���Na2CO3��NaCl��Na2SO4��CuCl2��BaCl2�е�һ�ֻ��֣��Ȱ����в������ʵ�飺���÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ���������˳��ij����м���ϡ������в��ֳ����ܽ⣬ͬʱ������ɫ���壮��������ʵ��ش���������
��1��������һ������ ��һ�������� ����ȷ���Ƿ��� ��
��2�����ɰ�ɫ�����Ļ�ѧ����ʽΪ ��������ɫ����Ļ�ѧ����ʽΪ ��
��1��������һ������
��2�����ɰ�ɫ�����Ļ�ѧ����ʽΪ
���㣺����δ֪��ļ���
ר�⣺ʵ��̽�������ݴ�����
�������������ʵ����ʽ������е�����ͨ��ȷ�����ڵ������ų����ܹ�������ʣ�
���ݣ����÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ��������֪һ����CuCl2��һ����Ba��Cl��2�����ܴ��ں�̼���������������ʣ�
���ݣ����˳��ij����м���ϡ������в��ֳ����ܽ⣬ͬʱ������ɫ���壬�жϳ���һ�������ᱵ��̼�ᱵ����һ������Na2CO3��Na2SO4���ݴ˻ش�
���ݣ����÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ��������֪һ����CuCl2��һ����Ba��Cl��2�����ܴ��ں�̼���������������ʣ�
���ݣ����˳��ij����м���ϡ������в��ֳ����ܽ⣬ͬʱ������ɫ���壬�жϳ���һ�������ᱵ��̼�ᱵ����һ������Na2CO3��Na2SO4���ݴ˻ش�
���
�⣺���÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ���������ж�һ����CuCl2��һ����Ba��Cl��2����Ϊֻ�б����Ӳſ����γɳ�����ͬʱ���ܺ�̼����Ļ��������������ӵĻ����
���˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬ͬʱ������ɫ���壬˵������һ�������ᱵ��̼�ᱵ����ӦΪBa2++SO42-=BaSO4����Ba2++CO32-=BaCO3����BaCO3+2H+=Ba2++CO2��+H2O��
����֪����һ�����У�Ba��Cl��2��Na2CO3��Na2SO4��һ��������CuCl2������ȷ��NaCl�Ƿ���ڣ�
��1��������������֪����һ�����У�Ba��Cl��2��Na2CO3��Na2SO4��һ��������CuCl2������ȷ��NaCl�Ƿ���ڣ��ʴ�Ϊ��Ba��Cl��2��Na2CO3��Na2SO4��CuCl2��NaCl��
��2�����˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬˵������һ�������ᱵ��̼�ᱵ����ӦΪBa��Cl��2+Na2CO3=BaCO3��+2NaCl��Ba��Cl��2+Na2SO4=BaSO4��+2NaCl��ͬʱ������ɫ���壬��BaCO3+2HCl=BaCl2+CO2��+H2O���ʴ�Ϊ��Ba��Cl��2+Na2CO3=BaCO3��+2NaCl��Ba��Cl��2+Na2SO4=BaSO4��+2NaCl��BaCO3+2HCl=BaCl2+CO2��+H2O��
���˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬ͬʱ������ɫ���壬˵������һ�������ᱵ��̼�ᱵ����ӦΪBa2++SO42-=BaSO4����Ba2++CO32-=BaCO3����BaCO3+2H+=Ba2++CO2��+H2O��
����֪����һ�����У�Ba��Cl��2��Na2CO3��Na2SO4��һ��������CuCl2������ȷ��NaCl�Ƿ���ڣ�
��1��������������֪����һ�����У�Ba��Cl��2��Na2CO3��Na2SO4��һ��������CuCl2������ȷ��NaCl�Ƿ���ڣ��ʴ�Ϊ��Ba��Cl��2��Na2CO3��Na2SO4��CuCl2��NaCl��
��2�����˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬˵������һ�������ᱵ��̼�ᱵ����ӦΪBa��Cl��2+Na2CO3=BaCO3��+2NaCl��Ba��Cl��2+Na2SO4=BaSO4��+2NaCl��ͬʱ������ɫ���壬��BaCO3+2HCl=BaCl2+CO2��+H2O���ʴ�Ϊ��Ba��Cl��2+Na2CO3=BaCO3��+2NaCl��Ba��Cl��2+Na2SO4=BaSO4��+2NaCl��BaCO3+2HCl=BaCl2+CO2��+H2O��
���������⿼�������ʼ���ķ�����Ӧ�ã��ؼ�������ʵ�����������жϣ����ô��ڵ������ų������ڵ����ʣ�

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��1mol��Լ����6.02��1023���� |
| B������ӵ���������ֵԼ����6.02��1023 |
| C���Ƶ�Ħ����������ֵ��һ�������Ƶ����ԭ������ |
| D������Ħ�������ָ��λ���ʵ�����������ռ����� |
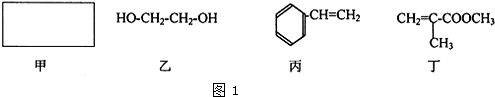
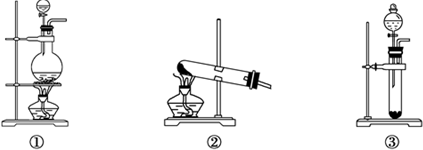
 ��Inorganic Syntheses�������ϳɣ�һ���У���һ��ͼ��ʾ��װ�ã������Ʊ�ij�ָ���Ĵ������壮��װ������װ��ҩƷ��ȷ���ǣ�������
��Inorganic Syntheses�������ϳɣ�һ���У���һ��ͼ��ʾ��װ�ã������Ʊ�ij�ָ���Ĵ������壮��װ������װ��ҩƷ��ȷ���ǣ�������| A��A��װŨ���ᣬB��װŨ���� |
| B��A��װŨ���ᣬB��װŨ���� |
| C��A��װ��������Ũ��Һ��B��װŨ��ˮ |
| D��A��װŨ��ˮ��B��װ��������Ũ��Һ |
�������и����������ʱ���ɰ��ܽ⡢���ˡ������IJ���˳����е��ǣ�������
| A���Ȼ��ƺ������ |
| B�����ۺ�п�� |
| C���Ȼ��غ�̼��� |
| D��̼��狀��Ȼ�� |
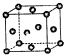 ��֪��X��Y��ZΪ������Ԫ�أ�X��Yͬ���ڣ�X��Zͬ���壬Yԭ�ӻ�̬ʱ��2p�����δ�ɶԵĵ�������࣬X�ĵͼ��������Y���ʷ��ӵĵ�������ȣ�W2+�ĺ�������Ų���ʽΪ��1S22S22P63S23P63d9��
��֪��X��Y��ZΪ������Ԫ�أ�X��Yͬ���ڣ�X��Zͬ���壬Yԭ�ӻ�̬ʱ��2p�����δ�ɶԵĵ�������࣬X�ĵͼ��������Y���ʷ��ӵĵ�������ȣ�W2+�ĺ�������Ų���ʽΪ��1S22S22P63S23P63d9��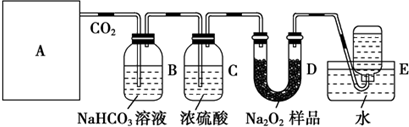
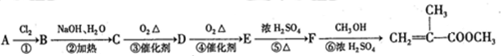 ��
��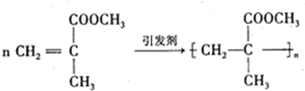
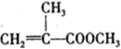 ���л������ĵ��壮��������������ϩ�����ͬ���칹�����
���л������ĵ��壮��������������ϩ�����ͬ���칹�����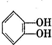 ����
����