��Ŀ����
��ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ���Ҫ�ɷ�ΪCuFeS2����������ʯ��Ϊ�ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺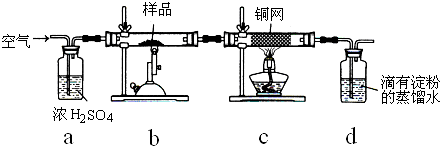
���õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��
������ƿ�У���0.05mol?L-1������Һ���еζ������ı�����Һ20.00mL����ش��������⣺
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ���� ��������ҺӦʢ���ڣ����ʽ������ʽ���� �ζ����У�
��2��װ��a�������� ��
A����ȥ�����еĶ�����̼��������������
B����ȥ�����е�ˮ����
C�������������ϡ�������������������������
D�������ڹ۲졢���ƿ�������
��3����ȥ��cװ�ã���ʹ�ⶨ������ƫ�͡�����ƫ�ߡ�����Ӱ�족�� ��д��Ӱ��ⶨ����Ļ�ѧ����ʽ ��
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���� ��
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ ��
��6������ʵ���������ȷ����õĻ�ͭ����Ȼƫ�ͣ����ܵ�ԭ����Ҫ�� ��
��7����װ�ô���һ�����Ե�ȱ�ݣ����Ը��� ��

���õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��
| 1 |
| 10 |
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ����
��2��װ��a��������
A����ȥ�����еĶ�����̼��������������
B����ȥ�����е�ˮ����
C�������������ϡ�������������������������
D�������ڹ۲졢���ƿ�������
��3����ȥ��cװ�ã���ʹ�ⶨ������ƫ�͡�����ƫ�ߡ�����Ӱ�족��
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ����
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ
��6������ʵ���������ȷ����õĻ�ͭ����Ȼƫ�ͣ����ܵ�ԭ����Ҫ��
��7����װ�ô���һ�����Ե�ȱ�ݣ����Ը���
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������ʵ��ԭ���ǣ���ͭ����Ʒ��bװ����ȼ�գ���ӦΪ8CuFeS2+21O2
8Cu+4FeO+2Fe2O3+16SO2�У�����Cu��Fe2O3��FeO��SO2���壬cװ�ó�ȥû��Ӧ����������ӦΪ2Cu+O2
2CuO��dװ�����շ�Ӧ�����Ķ�����������Ӧ��I2+SO2+2H2O=H2SO4+2HI��Ϊ�˱�֤ȫ�������գ�Ҫ��aװ��ͨ�������װ���ж��������ž������ݻ�ͭ�����ȷֽ�����Ķ�����������IJⶨ��������������õ�ˮ���궨�������Ԫ���غ����ȷ����ͭ����������������䴿�ȣ�
��1���������ı�������Լӿ컯ѧ��Ӧ���ʣ�����������ԣ�Ӧ������ʽ�ζ����У�
��2��Ũ������Խ�ˮ��ȥ�������Ը���ð�����ݵ����������ڿ������٣�
��3��ȥ��cװ�������ж���������ˮ��Һ�л��������Ӧ���ⶨ���ƫС��
��4����Ӧ�����Ķ�������Ӧ�þ����ܵı�dװ�����գ�
��5�����ҳ���ͭ��Ͷ������ⵥ�ʵĹ�ϵʽCuFeS2��2SO2��2I2���ٸ����������ݽ��м��㣻
��6���ⶨԭ���Ƿ�����Ӧ��I2+SO2+2H2O=H2SO4+2HI�����¶���������ٵIJ�����ʹ���ƫ�ͣ�
��7�����������ж�������ֱ���ŷŴ�����
| ||
| ||
��1���������ı�������Լӿ컯ѧ��Ӧ���ʣ�����������ԣ�Ӧ������ʽ�ζ����У�
��2��Ũ������Խ�ˮ��ȥ�������Ը���ð�����ݵ����������ڿ������٣�
��3��ȥ��cװ�������ж���������ˮ��Һ�л��������Ӧ���ⶨ���ƫС��
��4����Ӧ�����Ķ�������Ӧ�þ����ܵı�dװ�����գ�
��5�����ҳ���ͭ��Ͷ������ⵥ�ʵĹ�ϵʽCuFeS2��2SO2��2I2���ٸ����������ݽ��м��㣻
��6���ⶨԭ���Ƿ�����Ӧ��I2+SO2+2H2O=H2SO4+2HI�����¶���������ٵIJ�����ʹ���ƫ�ͣ�
��7�����������ж�������ֱ���ŷŴ�����
���
�⣺��1������Ʒ��ϸ���ٷ�Ӧ�����������ı������Ŀ����ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ�����������ԣ�Ӧ������ʽ�ζ����У�
�ʴ�Ϊ��ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ���ʽ��
��2��װ��a�е�Ũ����������տ����е�ˮ��������ֹˮ�������뷴Ӧװ��b�з���Σ�գ�ͬʱ����ð�������ݵĿ��������������ͨ������
��ѡBD��
��3��ȥ��cװ�������ж���������ˮ��Һ�л��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2SO2+O2+H2O=2H2SO4���ⶨ���ƫС��
�ʴ�Ϊ��ƫ�ͣ�2SO2+O2+H2O=2H2SO4��
��4����ͭ�����ȷֽ����ɶ��������һϵ�в���ֽ���Ϻ���Ȼ��Ҫͨ��һ��ʱ��Ŀ��������Խ�b��dװ���еĶ�������ȫ���ų�ȥ��ʹ������Ӿ�ȷ��
�ʴ�Ϊ��ʹ��Ӧ���ɵ�SO2ȫ������dװ���У�ʹ�ඨ�����ȷ��
��5��������ԭ���غ�͵����غ��ҳ���ϵʽ��CuFeS2��2SO2��2I2�����ĵ�0.05mol/L������Һ20.00mLʱ�������ĵĵⵥ�ʵ���Ϊ��0.05mol/L��0.02L=0.0010mol�����Ի�ͭ��������ǣ�0.5��0.0010mol��184g/mol��10=0.92g�������䴿���ǣ�
��100%=80%���ʴ�Ϊ��80%��
��6����������û�͵ⷴӦ���ݳ�����װ����������c��û��������d�кͶ�������Ӧ������ʹ���ƫ�ͣ�
�ʴ�Ϊ����ˮ����SO2����֣�H2SO3���ֱ�������
��7����������Ϊ�ж����壬����ֱ���ŷŵ������У�Ӧ����β������װ�ã��ʴ�Ϊ������β������װ�ã���ֹ��Ⱦ������
�ʴ�Ϊ��ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ���ʽ��
��2��װ��a�е�Ũ����������տ����е�ˮ��������ֹˮ�������뷴Ӧװ��b�з���Σ�գ�ͬʱ����ð�������ݵĿ��������������ͨ������
��ѡBD��
��3��ȥ��cװ�������ж���������ˮ��Һ�л��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2SO2+O2+H2O=2H2SO4���ⶨ���ƫС��
�ʴ�Ϊ��ƫ�ͣ�2SO2+O2+H2O=2H2SO4��
��4����ͭ�����ȷֽ����ɶ��������һϵ�в���ֽ���Ϻ���Ȼ��Ҫͨ��һ��ʱ��Ŀ��������Խ�b��dװ���еĶ�������ȫ���ų�ȥ��ʹ������Ӿ�ȷ��
�ʴ�Ϊ��ʹ��Ӧ���ɵ�SO2ȫ������dװ���У�ʹ�ඨ�����ȷ��
��5��������ԭ���غ�͵����غ��ҳ���ϵʽ��CuFeS2��2SO2��2I2�����ĵ�0.05mol/L������Һ20.00mLʱ�������ĵĵⵥ�ʵ���Ϊ��0.05mol/L��0.02L=0.0010mol�����Ի�ͭ��������ǣ�0.5��0.0010mol��184g/mol��10=0.92g�������䴿���ǣ�
| 0.92g |
| 1.15g |
��6����������û�͵ⷴӦ���ݳ�����װ����������c��û��������d�кͶ�������Ӧ������ʹ���ƫ�ͣ�
�ʴ�Ϊ����ˮ����SO2����֣�H2SO3���ֱ�������
��7����������Ϊ�ж����壬����ֱ���ŷŵ������У�Ӧ����β������װ�ã��ʴ�Ϊ������β������װ�ã���ֹ��Ⱦ������
���������⿼����̽����ͭ��Ĵ��ȣ��漰������ѡ���ȼ����֪ʶ��ע���ϵ���͵��ӵ�ʧ�غ��Ӧ�ã������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
������Ԫ��aW��bX��cY��dZ��ԭ����������������b-a=d-c��aW��cY��dZ��bX �������AB2���ۻ��������������Ͳ���ȫ��ͬ�����������в���ȷ���ǣ�������
| A��WZ2����������ԭ������㶼Ϊ8���ӽṹ |
| B��WX2��ZX2�Ļ�ѧ�����ͺ;������Ͷ���ͬ |
| C��4��Ԫ�ص���̬�⻯����X����̬�⻯�����ȶ� |
| D��ԭ�Ӱ뾶��С˳��ΪX��W��Y��Z |
�����������ڵ���ʵ��ǣ�������
| A��S |
| B��Cl2 |
| C��NaOH |
| D��CH4 |
 W��X��Y��Z�����ֳ����Ķ���������Ԫ�أ���ԭ�Ӱ뾶��ԭ�������ı仯��ͼ��ʾ����֪Y��Z����Ԫ�صĵ����ǿ�������Ҫ�ɷ֣�Wԭ�ӵ�������������Arԭ�ӵ��������������1������˵����ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ���������Ԫ�أ���ԭ�Ӱ뾶��ԭ�������ı仯��ͼ��ʾ����֪Y��Z����Ԫ�صĵ����ǿ�������Ҫ�ɷ֣�Wԭ�ӵ�������������Arԭ�ӵ��������������1������˵����ȷ���ǣ�������| A����һ�����ܣ�Z��Y |
| B��Y��W������⻯���ˮ��Һ�������� |
| C��W���ʵ�ˮ��Һ����Ư���� |
| D��W�ĵ��ʿɴ�Z�ļ��⻯�����û���Z�ĵ��� |
