��Ŀ����
17��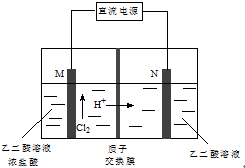 ��ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��壮��ҵ���á�˫���ҳɶԵ�ⷨ��������ȩ�ᣬԭ����ͼ��ʾ����װ��������������Ϊ���Ե缫�������Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᣮ����˵����ȷ���ǣ�������
��ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��壮��ҵ���á�˫���ҳɶԵ�ⷨ��������ȩ�ᣬԭ����ͼ��ʾ����װ��������������Ϊ���Ե缫�������Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᣮ����˵����ȷ���ǣ�������| A�� | M����ֱ����Դ�ĸ������� | |
| B�� | ����2 molH+ͨ�����ӽ���Ĥ����ȫ���뷴Ӧ�����װ�������ɵ���ȩ��Ϊ1 mol | |
| C�� | N�缫�ϵĵ缫��Ӧʽ��HOOC-COOH-2e-+2H+=HOOC-CHO+H2O | |
| D�� | �Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ��Ļ�ѧ����ʽ��Cl2+OHC-CHO+H2O=HOOC-CHO+2HCl |
���� A���������ӵ��ƶ�����ȷ��M�缫��������
B��2mol H+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽ���㣻
C��N�缫��HOOC-COOH�õ�������HOOC-CHO��
D���������������ԣ��ܽ�ȩ������Ϊ�Ȼ����ݴ���д����ʽ���ɣ�
��� �⣺A���������ӵ��ƶ�����ȷ��M�缫��������M����ֱ����Դ�ĸ�����������A����
B��2mol H+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽHOOC-COOH+2e-+2H+�THOOC-CHO+H2O����֪����1mol��ȩ�ᣬ��������������ȩ�������������ɵ���ȩ��Ϊ2mol����B����
C��N�缫��HOOC-COOH�õ�������HOOC-CHO����缫��ӦʽΪHOOC-COOH+2e-+2H+�THOOC-CHO+H2O����C����
D���������������ԣ��ܽ�ȩ������Ϊ�Ȼ����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ��Ļ�ѧ����ʽΪ��Cl2+OHC-CHO+H2O�THOOC-CHO+2HCl����D��ȷ��
��ѡD��
���� ���⿼���˵���ԭ���ķ���Ӧ�ã����յ���ԭ���Լ��������е����غ�ļ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
5�������µ���ƿ������Һ����һƿ������Һ��Ũ��ΪC1���ڶ�ƿ������Һ��Ũ��ΪC2���ڶ�ƿ������Һ�д�������ӵ�Ũ��ǡ��ΪC1�����Ȼ�У�������
| A�� | C1��C2 | |
| B�� | ��ƿ��Һ�ĵ���������ͬ | |
| C�� | ��һƿ��Һ��pH���ڵڶ�ƿ��Һ��pH | |
| D�� | ��һƿ��Һ�д���ĵ���̶�С�ڵڶ�ƿ��Һ�д���ĵ���̶� |
12����ϡ���ᡢŨ���ᡢ��ˮ�ֱ������ɫʯ����ֽ�ϣ������ֽ���ֵ���ɫ�����ǣ�������
| A�� | ��ɫ����ɫ����ɫ | B�� | ��ɫ����ɫ����ɫ | C�� | ��ɫ����ɫ����ɫ | D�� | ��ɫ����ɫ����ɫ |
2��0.1mol•L-1HF��Һ��pH=2�������Һ���й�Ũ�ȹ�ϵʽ����ȷ���ǣ�������
| A�� | c��H+����c��F-�� | B�� | c��H+����c��HF�� | C�� | c��OH-����c��HF�� | D�� | c��HF����c��F-�� |
9�������������� �ڹ��ۻ�������ǣ�������
| A�� | Na2O | B�� | NaCl | C�� | H2O | D�� | NaOH |
7������Ƭ�ӵ�1L0.5mol/L���Ȼ�����Һ�У��������Ӻ��������ӵ�Ũ����ͬʱ����Ƭ�����������ˣ�������
| A�� | 1.4g | B�� | 2.8g | C�� | 5.6g | D�� | 11.2g |
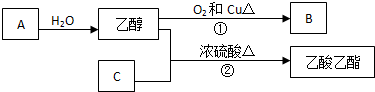
 ��ش��������⣺
��ش��������⣺