��Ŀ����
 ���ڹ�ҵ��չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1396��֣�2006�깤ҵSO2���ŷ����ﵽ��3800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1100��Ԫ��
���ڹ�ҵ��չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1396��֣�2006�깤ҵSO2���ŷ����ﵽ��3800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1100��Ԫ����1��д��������ҵ���������в���SO2��ʵ����
��
��2������SO2��Ⱦ�ɲ��õĴ�ʩ�У�д�����֣���
��
��3��ʪʽʯ��ʯ-ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯
Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������Һ�Ӵ���ͨ�����������ʯ�ࣨCaSO4?2H2O�����������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����� ����
��д��ʪ��ʯ��ʯ-ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�
�����������еõ���ʯ�࣬������Ȼ������Ҫ��Դ��ȼ��ú���������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����
��4��ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ�����ɣ�CaSO4?xH2O�����ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ��ͼ��ʾ�����ݱ��������������Ϊ2.72g���ٸı䣮��
��ʯ��Ļ�ѧʽ
��ͼ����AB�ζ�Ӧ������Ļ�ѧʽ
���㣺��������Ļ�ѧ����,�����������Ⱦ������
ר�⣺����Ԫ��
��������1����2������Ŀ���ȼ�ջ��ߺ����ʯ��ұ��������������������ݼ��ٶ���������ŷ����ַ�������SO2��Ⱦ�ɲ��õĴ�ʩ��
��3����ʪ��ʯ��ʯ-ʯ�෨���ù����У�����������������Ʒ�Ӧ����������ƣ�������Ʋ��ȶ����ױ�����������������������ƣ�
�����������ɱ��;���Ч����
�����������ˮ��
��4�����������֪����ǰ�ͼ��Ⱥ������ļ�������������ˮ����������ʵ������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����֪����ˮ������Ϊ��3.44g-2.72g=0.72g����ˮ������������Ƶ��������������ʯ��Ļ�ѧʽ���ٸ���A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g������ͼ���в���AB�ε�ԭ����ʯ�������Ϊ2.90g������CaSO42.72g��H2O Ϊ0.18g����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O���ݴ˽��
��3����ʪ��ʯ��ʯ-ʯ�෨���ù����У�����������������Ʒ�Ӧ����������ƣ�������Ʋ��ȶ����ױ�����������������������ƣ�
�����������ɱ��;���Ч����
�����������ˮ��
��4�����������֪����ǰ�ͼ��Ⱥ������ļ�������������ˮ����������ʵ������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����֪����ˮ������Ϊ��3.44g-2.72g=0.72g����ˮ������������Ƶ��������������ʯ��Ļ�ѧʽ���ٸ���A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g������ͼ���в���AB�ε�ԭ����ʯ�������Ϊ2.90g������CaSO42.72g��H2O Ϊ0.18g����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O���ݴ˽��
���
�⣺��1����2����ҵ���������в��������ʯ��ȼ�պ�ұ�����ŷŶ����������Ҫ;����Ҫ���ٶ����������Ⱦ��Ӧ�Ӽ��ٶ���������ŷ����֣����Բ�ȡ�Ĵ�ʩ�У����ٻ�ʯȼ�ϵ�ȼ�գ�����ʹ�������Դ�����ú��ȼ�������ʣ������������ȣ�
�ʴ�Ϊ���ٻ�ʯȼ�ϵ�ȼ�գ� �ں����ʯ��ұ����
��2���ټ��ٻ�ʯȼ�ϵ�ȼ�գ�����ʹ�������Դ�������ú��ȼ�������ʣ��۽����������ȣ�
��3���ٶ���������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
�ʴ�Ϊ��SO2+CaCO3=CaSO3+CO2��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
��ʯ��ʯ��Һ�ļ۸�ͣ��ʴ�Ϊ����ʯ��ʯ��Һ�ijɱ��ϵͣ�
�����������ˮ���Ȼ���������ˮ���ʴ�Ϊ����ˮϴ�ӣ�
��4������ʯ����ȷֽ���ٵ���������ˮ����������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����
CaSO4?xH2O�TCaSO4+xH2O
136 18x
2.72 0.72
���x=2 ����ʯ��Ļ�ѧʽΪCaSO4?2H2O��
�ʴ�Ϊ��CaSO4?2H2O��
����ʵ������֪��A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2OΪ2.90g-2.72g=0.18g��CaSO4�����ʵ���Ϊ
=0.02mol��H2O�����ʵ���Ϊ
=0.01mol����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O���ʴ�Ϊ��2CaSO4?H2O��
�ʴ�Ϊ���ٻ�ʯȼ�ϵ�ȼ�գ� �ں����ʯ��ұ����
��2���ټ��ٻ�ʯȼ�ϵ�ȼ�գ�����ʹ�������Դ�������ú��ȼ�������ʣ��۽����������ȣ�
��3���ٶ���������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
�ʴ�Ϊ��SO2+CaCO3=CaSO3+CO2��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
��ʯ��ʯ��Һ�ļ۸�ͣ��ʴ�Ϊ����ʯ��ʯ��Һ�ijɱ��ϵͣ�
�����������ˮ���Ȼ���������ˮ���ʴ�Ϊ����ˮϴ�ӣ�
��4������ʯ����ȷֽ���ٵ���������ˮ����������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����
CaSO4?xH2O�TCaSO4+xH2O
136 18x
2.72 0.72
���x=2 ����ʯ��Ļ�ѧʽΪCaSO4?2H2O��
�ʴ�Ϊ��CaSO4?2H2O��
����ʵ������֪��A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2OΪ2.90g-2.72g=0.18g��CaSO4�����ʵ���Ϊ
| 2.72g |
| 136g/mol |
| 0.18g |
| 18g/mol |
���������⿼��ú���ۺ����ã��漰����������Ի�������Ⱦ�����Σ�������ԭ����ʽ����д�ͽᾧˮ������жϣ���Ŀ�Ѷ��еȣ�����Ĺؼ�����ȷ���ʵ����ʣ�ע�⣨4����Ϊ�״��㣮

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
T��ʱ�������ȵļס��ҡ���3���ܱյ������У�������Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g������ʼʱ��������ʵ����ʵ������±���ʾ��������
��ﵽƽ��ʱ��CO�����ʵ����ɴ�С��˳���ǣ�
| CO2 | H2��g�� | CO��g�� | H2O��g�� | |
| �� | a mol | a mol | 0 | 0 |
| �� | a mol | 2a mol | 0 | 0 |
| �� | a mol | a mol | 0 | a mol |
| A��n���ף���n���ң���n������ |
| B��n���ף���n��������n���ң� |
| C��n���ң���n��������n���ף� |
| D��n���ң���n���ף���n������ |
����˵����ȷ���ǣ�������
| A����pH=5��������Һϡ��500����Һ��c��SO42-����c��H+����1��10 |
| B�������£���pH=5�Ĵ�����Һϡ��100������ҺpH=7 |
| C�������£���pH=9������������Һϡ��100������ҺpH=7 |
| D�������£���0.01mol?L-1��������Һϡ��100������ҺpH=4 |
������ɻ����ص��������ʱ�����ڿ����ľ���Ħ�����ɻ���˵��¶ȿɴ�1000�����ϣ�Ϊ�˷�ֹ���½����ջ٣���ѧ�Ҹ�����ɻ�����Ӧ��λ��װ�˿ɿ��ı����㣮����������IJ����ǣ�������
| A������ |
| B���ϳ���ά |
| C�������մɺ�̼��ά�ĸ��ϲ��� |
| D��þ���Ͻ� |
��ѧ�����������а�������Ҫ��ɫ������˵����Ӧ����ȷ���ǣ�������
| A��������Ӧָ�������ʷ�������ˮ�� |
| B�����ά�ͺϳ���ά�������л��߷��Ӳ��� |
| C���������͡���ˮ�Ҵ����Ǵ����� |
| D��ú��������Һ��������ѧ�仯���̣��ɱ�Ϊ�����Դ |
����˵������ȷ���ǣ�������
| A��һ������������1L������ͨ��46gNO2����NO2 �����ʵ���Ũ��һ��Ϊ1mol/L |
| B�������22.4L�ļ�������20NAԭ�� |
| C��1mol��������ˮת��1mol e- |
| D��1molNa2O2������ˮ��Ӧ��ת��1mol e-������0.5molO2 |
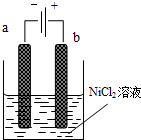 ������������Ni2O3��������������ܵ�أ�һ���Ʊ�Ni2O3�ķ����ǵ��NiCl2��Ni3+���ٽ�Ni3+��һϵ�з�Ӧ��ת��ΪNi2O3�������Ʊ��������£���NaOH��NiCl2��ҺpH��7.5���ӷ����������ƺ���е�⣮�������в�����Cl2������������������ClO-���Ѷ���������Ϊ����������ͼΪ���װ��ʾ��ͼ�����������������ӽ���Ĥ��������ش��������⣺
������������Ni2O3��������������ܵ�أ�һ���Ʊ�Ni2O3�ķ����ǵ��NiCl2��Ni3+���ٽ�Ni3+��һϵ�з�Ӧ��ת��ΪNi2O3�������Ʊ��������£���NaOH��NiCl2��ҺpH��7.5���ӷ����������ƺ���е�⣮�������в�����Cl2������������������ClO-���Ѷ���������Ϊ����������ͼΪ���װ��ʾ��ͼ�����������������ӽ���Ĥ��������ش��������⣺ ���ܱ���������A��B�������ʣ���һ���������·�Ӧ��2A������+B���̣�?2C��������H��0�ﵽƽ��ı�һ��������X������������Y��һ������ͼ�����ߵ��ǣ�������
���ܱ���������A��B�������ʣ���һ���������·�Ӧ��2A������+B���̣�?2C��������H��0�ﵽƽ��ı�һ��������X������������Y��һ������ͼ�����ߵ��ǣ������� ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ���ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ���ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����