��Ŀ����
6��A����Ȼ������Ҫ�ɷ֣���AΪԭ����һ�������¿ɻ���л���B��C��D��E��F�����ת����ϵ��ͼ����֪��B�ڱ�״���µ��ܶ�Ϊ1.16g•L-1��C�ܷ���������Ӧ��FΪ��Ũ����ζ����������ˮ����״Һ�壮
��ش�
��1���л���D�к��еĹ������������Ȼ���
��2��D+E��F�ķ�Ӧ������������Ӧ��
��3���л���A�ڸ�����ת��ΪB�Ļ�ѧ����ʽ��2CH4$\stackrel{����}{��}$CH��CH+3H2��
��4������˵����ȷ����BD��
A���л���E������Ʒ�Ӧ��ˮ������Ʒ�ӦҪ����
B���л���D��E��F���ñ���Na2CO3��Һ����
C��ʵ�����Ʊ�Fʱ��Ũ������Ҫ����������
D���л���C�ܱ����Ƽ���������ͭ����Һ������KMnO4��Һ������
���� A����Ȼ������Ҫ�ɷ֣���AΪCH4����֪��B�ڱ�״���µ��ܶ�Ϊ1.16g•L-1����B��Ħ������Ϊ��M��B��=22.4L/mol��1.16g•L-1��26g/mol��C�ܷ���������Ӧ����C�����к���ȩ����FΪ��Ũ����ζ����������ˮ����״Һ�壬��FΪ��������ת����ϵ��֪FΪCH3COOC2H5��C��������D��C������������ԭ��Ӧ����E����DΪCH3COOH��EΪCH3CH2OH��CΪCH3CHO��B��ˮ��Ӧ������ȩ����B��Ħ������Ϊ26g/mol����BΪCH��CH���ݴ˽��н��
��� �⣺A����Ȼ������Ҫ�ɷ֣���AΪCH4����֪��B�ڱ�״���µ��ܶ�Ϊ1.16g•L-1����B��Ħ������Ϊ��M��B��=22.4L/mol��1.16g•L-1��26g/mol��C�ܷ���������Ӧ����C�����к���ȩ����FΪ��Ũ����ζ����������ˮ����״Һ�壬��FΪ��������ת����ϵ��֪FΪCH3COOC2H5��C��������D��C������������ԭ��Ӧ����E����DΪCH3COOH��EΪCH3CH2OH��CΪCH3CHO��B��ˮ��Ӧ������ȩ����B��Ħ������Ϊ26g/mol����BΪCH��CH��
��1��D�Ľṹ��ʽΪDΪCH3COOH���京�й�����Ϊ�Ȼ���
�ʴ�Ϊ���Ȼ���
��2��DΪCH3COOH��EΪCH3CH2OH��������Ũ������������·���������Ӧ��������������
�ʴ�Ϊ��������Ӧ��
��3��AΪCH4��BΪCH��CH��CH4�ڸ�����ת��ΪCH��CH�Ļ�ѧ����ʽ�ǣ�2CH4$\stackrel{����}{��}$CH��CH+3H2��
�ʴ�Ϊ��2CH4$\stackrel{����}{��}$CH��CH+3H2��
��4��A��EΪCH3CH2OH���Ҵ�������Ʒ�Ӧ����ˮ������Ʒ�Ӧ���ң���A����
B��DΪCH3COOH��EΪCH3CH2OH��FΪCH3COOC2H5��������̼�����������壬�������������ڱ���̼������Һ���Ҵ�������̼������Һ�����Կ��ñ���Na2CO3��Һ�������ߣ���B��ȷ��
C��ʵ�����Ʊ���������ʱ��Ũ����Ϊ��������ˮ���������������ԣ���C����
D���л���CΪCH3CHO��CH3CHO�к��й�����ȩ����ȩ���ܱ����Ƽ���������ͭ����Һ������KMnO4��Һ��������D��ȷ��
�ʴ�Ϊ��BD��
���� ���⿼���л��ϳɣ���Ŀ�ѶȲ����ƶ�������ɡ��ṹΪ���ؼ���ע���������ճ����л�����ɡ��ṹ�����ʣ��������������ѧ���ķ���������������������

| A�� | ��HA��HB��Ϊ���ᣬ���ԣ�HA��HB������ͬ�����£���Һ��pH��СΪNaA��NaB | |
| B�� | ��ͬ�����£���pH=11��NaOH��Һ�ͺͰ�ˮ�ֱ�ϡ��pH=9����Һ������ˮ�����ǰ�ߴ� | |
| C�� | pH=3�Ĵ�����pH=1��NaOH��Һ��������ʱ����Һ������Ũ�ȵĴ�С˳���ǣ�c��Na+����c��CHCOO-����c��OH-����c��H+�� | |
| D�� | 0.1mol/L��ij��Ԫ������Na2A��Һ�У�c��Na+��=2[c��HA-��+c��A2-��+c��H2A��] |
| A�� | ��ԭ�ӵĽṹʾ��ͼ��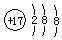 | B�� | ������ӵ����ģ�ͣ� | ||
| C�� | �Ȼ�þ�ĵ���ʽ��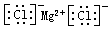 | D�� | ԭ�Ӻ�����8�����ӵ���ԭ�ӣ�818O |
| A�� | ��NaOH��Һ�е��뼸��NH4Cl��Һ��NH4++OH-�TNH3��+H2O | |
| B�� | ����ͨ��ˮ�У�2F2+2H2O�T4H++4F-+O2 | |
| C�� | AlCl3��Һ��ͨ������İ�����Al3++3NH3•H2O�TAl��OH��3��+3NH${\;}_{4}^{+}$ | |
| D�� | ������ͭм����Ũ�����У�Cu+4 H++2NO3-�TCu2++2 NO2��+2 H2O |
 ��
��