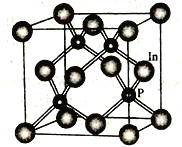
��Ŀ����
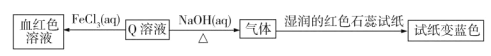
����Ŀ����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹʪ��ĺ�ɫʯ����ֽ���������壬A�� B������β������Ҫ�ɷ֣�����֮���ܷ������·�Ӧ��
��![]() ��
��
��![]()
![]() ����
����![]() ��
��
��![]()
(1)д�����ǵĻ�ѧʽ��A______��C______��D______��F______��
(2)д��![]() ��Ӧ�Ļ�ѧ����ʽ������Ҫ����գ�_______________________________�������Ӧ______
��Ӧ�Ļ�ѧ����ʽ������Ҫ����գ�_______________________________�������Ӧ______![]() ����������������������
����������������������![]() ������ԭ��Ӧ��
������ԭ��Ӧ��
(3)��ҵ����C�Ĺ�������һ����ӦΪF������������B��![]() ��д���ò���Ӧ�Ļ�ѧ����ʽ��________________________________��
��д���ò���Ӧ�Ļ�ѧ����ʽ��________________________________��
���𰸡�NO2 HNO3 NH4NO3 NH3 NH3+HNO3=NH4NO3 ������ 4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
��������
F��ʹʪ��ĺ�ɫʯ����ֽ���������壬��FΪNH3���ɢ�![]() ��DӦΪ��Σ���E��Ҳ���е�Ԫ�أ���EΪNaNO3����DΪNH4NO3���ɢ�
��DӦΪ��Σ���E��Ҳ���е�Ԫ�أ���EΪNaNO3����DΪNH4NO3���ɢ�![]() ��CӦΪHNO3��A�� B������β������Ҫ�ɷ֣���A��B�ֱ�ΪNO2��NO��
��CӦΪHNO3��A�� B������β������Ҫ�ɷ֣���A��B�ֱ�ΪNO2��NO��
������Ӧ����ʽ�ֱ�Ϊ����![]() ����
����![]() ����
����![]() ��
��
(1)�����Ϸ�����֪��A��C��D��F�ֱ�Ϊ��NO2��HNO3��NH4NO3��NH3��
(2)�ڵĻ�ѧ����ʽΪ��![]() ����Ӧǰ��û�л��ϼ۱仯���ʲ�����������ԭ��Ӧ��
����Ӧǰ��û�л��ϼ۱仯���ʲ�����������ԭ��Ӧ��
(3)F��NH3��BΪNO��������������������NO��H2O����Ӧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�