��Ŀ����
13�� ������ѧ���ش��������⣺
������ѧ���ش��������⣺��1����������Һ��ͨ������CO2C6H5ONa+CO2+H2O��C6H6OH+NaHCO3
�ڼ�ȩ�����Ƶ�Cu��OH��2��ӦHCHO+4Cu��OH��2$\stackrel{��}{��}$2Cu2O��+5H2O+CO2��
��ij�л���Ľṹ��ʽ��ͼ��ʾ��1mol���л���������NaOH��Һ���ȣ���ַ�Ӧ����������NaOH�����ʵ���Ϊ8 mol
��2�������Ŷ��л���������������ã���Ҳ���ܵ��������ŵ�Ӱ�죮
�ٱȽϷе�
 ��
��  ���������������=������ͬ��
���������������=������ͬ���ڱȽ�ˮ���ԣ�
 ��
��
�۱Ƚ����ԣ�
 ��CH3COOH��
��CH3COOH��
���� ��1���ٱ����ƺͶ�����̼��ˮ��Ӧ���ɱ��Ӻ�̼�����ƣ�
�ڼ�ȩ������������ͭ����Һ����������Ӧ���ɶ�����̼��ˮ�� ������ͭ��
�۸÷������ܺ�NaOH��Ӧ�����Ȼ�������ˮ�����ɵ��Ȼ������ǻ���±��ԭ�ӣ�
��2����̼ԭ�Ӹ�����ͬ�Ĵ��д��ǻ�����Խ�࣬���۷е�Խ�ߣ�
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������
��� �⣺��1���ٱ����ƺͶ�����̼��ˮ��Ӧ���ɱ��Ӻ�̼�����ƣ���Ӧ����ʽΪC6H5ONa+CO2+H2O��C6H6OH+NaHCO3��
�ʴ�Ϊ��C6H5ONa+CO2+H2O��C6H6OH+NaHCO3��
�ڼ�ȩ������������ͭ����Һ����������Ӧ���ɶ�����̼��ˮ�� ������ͭ����Ӧ����ʽΪHCHO+4Cu��OH��2$\stackrel{��}{��}$2Cu2O��+5H2O+CO2����
�ʴ�Ϊ��HCHO+4Cu��OH��2$\stackrel{��}{��}$2Cu2O��+5H2O+CO2����
���ɽṹ��ʽ��֪�������к�-COOH��-Cl��-Br��-COOC-����NaOH��Һ��Ӧ�����뱽��ֱ��������-Cl��-Brˮ�����ɵķ�-OH��-COOC-ˮ�����ɵķ�-OHҲ��NaOH��Ӧ����1mol���л�����������NaOH��Һ���ȣ���ַ�Ӧ����������NaOH�����ʵ���Ϊ8mol��
�ʴ�Ϊ��8��
��2����̼ԭ�Ӹ�����ͬ�Ĵ��д��ǻ�����Խ�࣬���۷е�Խ�ߣ������۷е�ǰ��С�ں��ߣ��ʴ�Ϊ������
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�����ˮ���ԣ�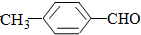 ��
��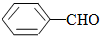 ���ʴ�Ϊ����������
���ʴ�Ϊ����������
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������ʹ����������ӱ���������Ӹ��ȶ����������Ա�����ǿ���ʴ�Ϊ������
���� ���⿼���л���ṹ�����ʣ�Ϊ��Ƶ���㣬��ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ������ؿ���ѧ�������жϼ�֪ʶǨ��������ע���ȩ������������ͭ��������Ӧ�������ɼ���������ɶ�����̼��Ϊ�״��㣮

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�| A�� | �з��ɽ���߷��Ӳ��ϣ����١���ɫ��Ⱦ�� | |
| B�� | ������ClO2������Ϊ��ˮ������ԭ����ȫ��ͬ | |
| C�� | ��ˮ������ҵ�����������ˮ���������ã��ǽ��ȱˮ�������Ч;�� | |
| D�� | Һ�ȹ�й©ʱ���ɽ�������ˮ���У�����ˮ���м�����ʯ�� |
| A�� | Ư����Ч�ɷֵĻ�ѧʽ��Ca��ClO��2 | |
| B�� | ������ĵ��뷽��ʽ��H2SO3�T2H++SO32- | |
| C�� | NO��NO2�������������� | |
| D�� | Na+�Ľṹʾ��ͼ�� |
��1�����Ƶ��̷���FeSO4•7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe��OH��SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ��4FeSO4•7H2O+O2=4Fe��OH��SO4+26H2O��
��2����֪FeSO4�ڲ�ͬ�����·ֽ�õ����ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��SO3�۵���16.8�棬�е���44.8�森
ij�о���ѧϰС����������װ�ý���ʵ��̽�����ڼ���������FeSO4�ķֽ�����

����װ�â�͢����������������Իش��������⣺
�٢�װ���ձ���ˮ���¶�Ӧ������ ��ѡ�0�桢25�桢50�桱����װ�â�������Ƿ�ֹ������������������ȫƿ������
��װ�â��е��Լ�������C ��ѡ����ţ���ͬ���������Dz�����ɫ��������֤����������к���SO3��װ�â��е��Լ�������B��E��
A.2mol/LNa2CO3��Һ B��Ʒ����Һ C.0.5mol/L BaCl2��Һ D.0.5mol/LBa��NO3��2 E.0.01mol/L KMnO4��Һ F�����۵⻯����Һ
��װ��V���Լ�ΪNaOH��Һ��������Ӧ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O��
��Ϊ�˼���������ɷ֣�ȡ��Ӧ��Ĺ������Թ��У���ϡ�����ܽ⣬��������Һ�ֳ����ݣ���������ʵ�飺
| �������� | Ԥ��ʵ������ | Ԥ��ʵ����� |
| ������һ����Һ�м���KSCN��Һ�������軯����Һ�� | ��Һ���Ѫ��ɫ | �����к���Fe2O3 |
| ����һ����Һ�еμ�2�λ�ɫK3[Fe��CN��6]��Һ | ������ɫ���� | �����к���FeO |
| A�� | ��⾫��ͭ������·��ͨ���ĵ�����Ϊ0.1NAʱ��������������һ��Ϊ3.2g | |
| B�� | 0.5L1mol•L-1NaHS����Һ�У�Na+��HS-������Ŀ֮��ΪNA | |
| C�� | 25��ʱ��7gC2H4��C2H6��������У�����NA��C-H�� | |
| D�� | ����£�11.2Lһ�ȼ����к��е���ԭ����Ϊ0.5NA |
| Ԫ�ر�� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-1nm�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 |
| ����ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| ��ͻ��ϼ� | -2 | -3 | -1 | -3 |
| A�� | Ԫ�آݵ��⻯��е����Ԫ�آٵ��⻯�� | |
| B�� | �ܴۢ���ͬһ���� | |
| C�� | ��������Ԫ���У�����������Ӧˮ�����������ǿ����Ԫ�آ� | |
| D�� | ���Ԫ��λ�����ڱ��е������ڵڢ�A�� |
 ����Ӧ���ͣ��Ӿ۷�Ӧ����ۺϷ�Ӧ��
����Ӧ���ͣ��Ӿ۷�Ӧ����ۺϷ�Ӧ�� ��Ӧ���ͣ�ȡ����Ӧ
��Ӧ���ͣ�ȡ����Ӧ Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣� ��
��