题目内容
|
肼(N2H4)是火箭发动机的一种燃料,反应时N2O4为氧化剂,反应生成N2和水蒸气.已知: N2(g)+2O2(g) ΔH=+8.7 kJ·mol-1 N2H4(g)+O2(g) ΔH=-534 kJ·mol-1 下列表示肼和N2O4反应的热化学方程式,正确的是 | |
| [ ] | |
A. |
2N2H4(g)+N2O4(g) ΔH=-1 076.7 kJ·mol-1 |
B. |
N2H4(g)+ ΔH=-1 076.7 kJ·mol-1 |
C. |
2N2H4(g)+N2O4(g) ΔH=-542.7 kJ·mol-1 |
D. |
2N2H4(g)+N2O4(g) ΔH=-1 059.3 kJ·mol-1 |
答案:A
解析:
解析:
|
N2(g)+2O2(g) N2H4(g)+O2(g) ②×2-①得2N2H4(g)+N2O4(g) |

练习册系列答案
 千里马走向假期期末仿真试卷寒假系列答案
千里马走向假期期末仿真试卷寒假系列答案
相关题目
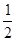 N2O4(g) ====
N2O4(g) ====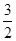 N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1
N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1