��Ŀ����
��(N2H4)�ǻ����������һ��ȼ�ϣ���ӦʱN2O4Ϊ������������N2��ˮ��������֪��N2(g)+2O2(g)![]() N2O4(g) ��H=+8.7 kJ��mol-1
N2O4(g) ��H=+8.7 kJ��mol-1
N2H4(g)+O2(g) ![]() N2(g)+2H2O(g) ��H=-534.0 kJ��mol-1
N2(g)+2H2O(g) ��H=-534.0 kJ��mol-1
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ���� ���� ��
A.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-542.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-542.7 kJ��mol-1
B.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-1 059.3 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 059.3 kJ��mol-1
C.N2H4(g)+![]() N2O4(g)
N2O4(g) ![]()
![]() N2(g)+2H2O(g) ��H=-1 076.7 kJ��mol-1
N2(g)+2H2O(g) ��H=-1 076.7 kJ��mol-1
D.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1
�����������������Ӧ�еĵڶ�����2�͵�һ����Ӧ��������ɵõ��º�������������Ӧ���Ȼ�ѧ����ʽ��2N2H4(g)+N2O4(g)![]() 3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1
3N2(g)+4H2O(g) ��H=-1 076.7 kJ��mol-1
�𰸣�D

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
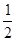 N2O4(g) ====
N2O4(g) ====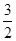 N2(g)+2H2O(g)��H="-1076.7" kJ��mol-1
N2(g)+2H2O(g)��H="-1076.7" kJ��mol-1