��Ŀ����
����Ŀ����Ԫ�����ֲ�����������������Ľ�������ʮ����Ҫ�����á���ش��������}��
��1�����־�����������ο�ѧ�ķ���Ϊ_________________________________��
��2������Bԭ�ӻ�̬�ļ۲�����Ų�ͼ����ȷ����___________
A. B.
B.
C. D.
D.
��3��NaBH4����Ҫ�Ĵ������壬�����ӵ����幹��Ϊ___________��
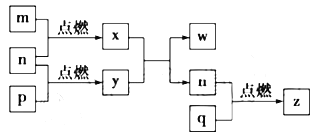
��4������������(�ṹ����ͼ1��ʾ)��___________����(�������Ǽ���)��
��5��ͼ2��ʾ����������ӵ�һ����������ʽ�ṹ���仯ѧʽ�ɱ�ʾΪ___________��

��6�����ᾧ����Ƭ��ṹ������һ��Ľṹ��ͼ3��ʾ����������ˮ���ܽ�Ⱥ�С��������ˮ�нϴ�ԭ����_________________________________��
��7������������(BN)������ǿ����ĥ���ϣ�����Ϊ��������ı����㣬�侧���ṹ(����ͼ)����ʯ���ơ���֪�þ����ܶ�Ϊag/cm3������������Nԭ�Ӽ����С����Ϊ___________pm��(�ú�a�Ĵ���ʽ��ʾ��NA��ʾ�����ӵ�����)

���𰸡�X-��������ʵ�� A �������� ���� BO2- �� (BO2)nn- �� BnO2nn- ������������Ӽ����������һ�������ܽ⣻����ʱ�������в���������ƻ������������ˮ�����γ�������ܽ������ ![]() ��1010
��1010
��������
��1�����־��������������ɿ��Ŀ�ѧ����Ϊ����X-��������ʵ�飻
��2��Bԭ����Χ������3�����ӣ�2s�ܼ�����2�����ӣ�2p�ܼ�����1�����ӣ�����Χ�����Ų�ͼΪ ����ѡA��
����ѡA��
��3��NaBH4��һ����Ҫ�Ĵ������壬�����ӵĻ�ѧʽΪBH4��������ṹʽΪ![]() �����幹��ΪΪ�������壻
�����幹��ΪΪ�������壻
��4��P4S3��Pԭ�ӳ�3��P-S��������һ�Թ¶Ե��ӣ��ӻ������Ϊ4��Pԭ�Ӳ�ȡsp3�ӻ������ڼ��Է��ӣ�
��5����ͼ�ɿ�����ÿ��BO32-��Ԫ������һ��B����һ��O��ȫ���������Ԫ��ʣ���2��O�ֱ�Ϊ2��BO32-��Ԫ���ã�����B��O=1����1+2��1/2��=1��2����ѧʽΪBO2-�� (BO2)nn- �� BnO2nn-��
��6�����ھ�����������Ӽ����������һ�������ܽ⣻����ʱ�������в���������ƻ����ܽ�������������ᾧ������ˮ���ܽ�Ⱥ�С��������ˮ�нϴ�
��7��һ��������Bԭ����Ŀ=![]() ����Nԭ����=4��һ������������
����Nԭ����=4��һ������������![]() ������
������![]() ��
��![]() �������ⳤΪ
�������ⳤΪ![]() ����ϸ�۲�BN�ľ����ṹ���ѷ���Nλ�ھ�����8��С�������л������ڵ�4��С����������ģ�N��֮�������Ǿ�����Խ��ߵ�һ�룬��˾��������������Nԭ�Ӽ�ľ���Ϊ��
����ϸ�۲�BN�ľ����ṹ���ѷ���Nλ�ھ�����8��С�������л������ڵ�4��С����������ģ�N��֮�������Ǿ�����Խ��ߵ�һ�룬��˾��������������Nԭ�Ӽ�ľ���Ϊ��![]() ��1010�����
��1010�����

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�����Ŀ������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݡ�
��ѧʽ | ����ƽ�ⳣ��(25��) |
HCN | K=4.9��10-10 |
CH3COOH | K=1.8��10-5 |
H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
(1)25 ��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ��������Һ��pH�ɴ�С��˳��Ϊ__________________��
(2)25 ��ʱ��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ��ϣ���������Һ�����ԣ���c(Na��)__________c(CH3COO��)(����>������<������=��)��
(3)NaCN��Һ��ͨ������CO2����������Ӧ�Ļ�ѧ����ʽΪ___________��
(4)25 ��ʱ�� pH=8��CH3COONa��Һ�У�c(Na��)-c(CH3COO-)=___________��