��Ŀ����
|
�״��㷺����ȼ�ϵ�ص�ȼ�ϣ�������Ȼ�����ϳɣ���֪�� �� 2CH4(g)��O2(g)�� CO(g)��2H2(g)�� CH4(g)��2O2(g)����˵�����ܵó��Ľ����� | |
| [����] | |
A�� |
������������Ӧ�У����Ƿ�Ӧ�������������������������� |
B�� |
CO(g)��2H2(g) |
C�� |
�״���ȼ����Ϊ 764 kJ/mol |
D�� |
�� CO��ȼ����Ϊ282.5 kJ/mol����H2��ȼ����Ϊ286 kJ/mol |
�𰸣�B
������
������
|
|

��ϰ��ϵ�д�
�����Ŀ
�״��㷺����ȼ�ϵ�ص�ȼ�ϣ�������Ȼ�����ϳɣ���֪��
��2CH4��g��+O2��g��=2CO��g��+4H2��g������H=-71kJ?mol-1
��CO��g��+2H2��g��=CH3OH��l������H=-90.5kJ?mol-1
��������������ǣ�������
��2CH4��g��+O2��g��=2CO��g��+4H2��g������H=-71kJ?mol-1
��CO��g��+2H2��g��=CH3OH��l������H=-90.5kJ?mol-1
��������������ǣ�������
| A��CO��g��+2H2��g���TCH3OH��g����H��-90.5kJ?mol-1 | ||
| B���ڼ״�ȼ�ϵ���У��״����ڵ缫Ϊ���� | ||
C��CH4��g��+
| ||
D��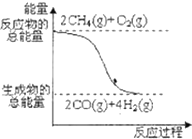 ��Ӧ���е������仯��ͼ��ʾ |
�״��㷺����ȼ�ϵ�ص�ȼ�ϣ�������Ȼ�����ϳɣ���֪��
��2CH4��g��+O2��g��=2CO��g��+4H2��g����H=-71kJ/mol
��CO��g��+2H2��g��=CH3OH��l����H=-90.5kJ/mol
��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890kJ/mol�����ܵó��Ľ����ǣ�������
��2CH4��g��+O2��g��=2CO��g��+4H2��g����H=-71kJ/mol
��CO��g��+2H2��g��=CH3OH��l����H=-90.5kJ/mol
��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890kJ/mol�����ܵó��Ľ����ǣ�������
| A�������ȼ���ȡ�H=-890kJ/mol | B����4NA��̼�����õ��Ӷ�����ʱ����Ӧ�۷ų�890kJ������ | C���״���ȼ���ȡ�H=-1528 kJ/mol | D����CO��ȼ���ȡ�H=-282.5 kJ/mol����H2��ȼ���ȡ�H=-286 kJ/mol |
