��Ŀ����
��ͼ����ѧ��ѧ�������ʵ�ת����ϵ��ijЩ��Ӧ���������ֲ�������ȥ����A��GΪ�ճ������еij���������B��C��E��I��JΪ���壬����CΪ����ɫ���壬JΪ����ɫ���壮DΪ��ɫ���壬MΪ���ɫ���壮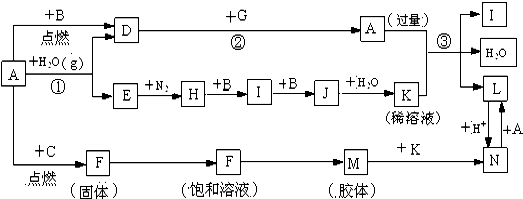
��ش��������⣺
��1��AԪ�������ڱ���λ�� ���� �壮
��2����F������Һ�Ʊ�M����IJ���Ϊ ��
��3������L��Һ��N��Һ��������������� ��
��4����Ӧ�۵����ӷ���ʽΪ ��
��5����֪����ͨ��״���£���Ӧ���У�1molG���ʷ�����Ӧ���ų�akJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ ��

��ش��������⣺
��1��AԪ�������ڱ���λ��
��2����F������Һ�Ʊ�M����IJ���Ϊ
��3������L��Һ��N��Һ���������������
��4����Ӧ�۵����ӷ���ʽΪ
��5����֪����ͨ��״���£���Ӧ���У�1molG���ʷ�����Ӧ���ų�akJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ
���㣺������ƶ�
ר�⣺�ƶ���
������B��C��E��I��JΪ���壬����CΪ����ɫ���壬��C��Cl2��JΪ����ɫ���壬��J��NO2��DΪ��ɫ���壬MΪ���ɫ���壬MΪFe��OH��3��A��GΪ�ճ������еij���������A�ܺ�ˮ������Ӧ����A��Fe������B��Ӧ����D��B�����壬��D��Fe3O4��BΪO2��E��H2��F��FeCl3��H��NH3��IΪNO��JΪNO2��KΪHNO3��G�������г����������ܷ����û���Ӧ����G��Al����������ϡ���ᷴӦ����L��L��Fe��NO3��2��������������������N��NΪFe��NO3��3�����Ԫ�ء����ʡ�������Ľṹ�����ʷ������
���
�⣺B��C��E��I��JΪ���壬����CΪ����ɫ���壬��C��Cl2��JΪ����ɫ���壬��J��NO2��DΪ��ɫ���壬MΪ���ɫ���壬MΪFe��OH��3��A��GΪ�ճ������еij���������A�ܺ�ˮ������Ӧ����A��Fe������B��Ӧ����D��B�����壬��D��Fe3O4��BΪO2��E��H2��F��FeCl3��H��NH3��IΪNO��JΪNO2��KΪHNO3��G�������г����������ܷ����û���Ӧ����G��Al����������ϡ���ᷴӦ����L��L��Fe��NO3��2��������������������N��NΪFe��NO3��3��
��1��A��Fe����Ԫ��λ�ڵ������ڵڢ��壬
�ʴ�Ϊ���ģ�����
��2���Ʊ�������������ķ���Ϊ��ȡһ���ձ�������20mL����ˮ���������У�Ȼ�����ˮ�еμ�FeCl3������Һ1mL��2mL��������У�����Һ�ʺ��ɫΪֹ��
�ʴ�Ϊ��ȡһ���ձ�������20mL����ˮ���������У�Ȼ�����ˮ�еμ�FeCl3������Һ1mL��2mL��������У�����Һ�ʺ��ɫΪֹ��
��3������������dz��ɫ���������Ի�ɫ�����Լ���Fe��NO3��2��Һ��Fe��NO3��3��Һ��������������ǹ۲���Һ����ɫ��
�ʴ�Ϊ���۲���Һ����ɫ��
��4����������ϡ���ᷴӦ��������������һ��������ˮ�����ӷ���ʽΪ3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
�ʴ�Ϊ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
��5����ͨ��״���£���Ӧ���У�1molAl���ʷ�����Ӧ���ų�akJ��������8molAl�μӷ�Ӧ�ų�������Ϊ8akJ��
�������Ȼ�ѧ��Ӧ����ʽΪ3Fe3O4��s��+8Al��s���T4Al2O3��s��+9Fe��s����H=-8akJ/mol��
�ʴ�Ϊ��3Fe3O4��s��+8Al��s���T4Al2O3��s��+9Fe��s����H=-8akJ/mol��
��1��A��Fe����Ԫ��λ�ڵ������ڵڢ��壬
�ʴ�Ϊ���ģ�����
��2���Ʊ�������������ķ���Ϊ��ȡһ���ձ�������20mL����ˮ���������У�Ȼ�����ˮ�еμ�FeCl3������Һ1mL��2mL��������У�����Һ�ʺ��ɫΪֹ��
�ʴ�Ϊ��ȡһ���ձ�������20mL����ˮ���������У�Ȼ�����ˮ�еμ�FeCl3������Һ1mL��2mL��������У�����Һ�ʺ��ɫΪֹ��
��3������������dz��ɫ���������Ի�ɫ�����Լ���Fe��NO3��2��Һ��Fe��NO3��3��Һ��������������ǹ۲���Һ����ɫ��
�ʴ�Ϊ���۲���Һ����ɫ��
��4����������ϡ���ᷴӦ��������������һ��������ˮ�����ӷ���ʽΪ3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
�ʴ�Ϊ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
��5����ͨ��״���£���Ӧ���У�1molAl���ʷ�����Ӧ���ų�akJ��������8molAl�μӷ�Ӧ�ų�������Ϊ8akJ��
�������Ȼ�ѧ��Ӧ����ʽΪ3Fe3O4��s��+8Al��s���T4Al2O3��s��+9Fe��s����H=-8akJ/mol��
�ʴ�Ϊ��3Fe3O4��s��+8Al��s���T4Al2O3��s��+9Fe��s����H=-8akJ/mol��
���������⿼������������������Ʊ������ȷ�Ӧ��Ԫ��λ�õ��жϵ�֪ʶ�㣬��ȷ�ƶ������ǽⱾ��ؼ�����C��J��MΪͻ�ƿڣ������������ϵķ��������ƶϣ�ע�⣺�������������Ʊ������в����ò��������裬���ܳ�ʱ����к��ɫҺ�壬��������������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
�����йص������Һ��������ȷ���ǣ�������
| A��ͬŨ�ȡ�ͬ�����ǿ����ǿ����Һ��Ϻ���Һһ��Ϊ���� |
| B����Na2S��Һ�У�c��Na+��=2[c��S2-��+c��HS-��+c��H2S��] |
| C����1 mol KOH����Һ��1 mol CO2��ȫ��Ӧ����Һ��c��K+��=c��HCO3-�� |
| D����CH3COONa��Һ�м�������CH3COOH����ʹc��Na+��=c��CH3COO-�� |
�ڳ��£�25�棩ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
����˵��������ǣ�������
| ���� | X | Y | Z |
| ��ʼŨ��/mol?L-1 | 0.1 | 0.2 | 0 |
| ƽ��Ũ��/mol?L-1 | 0.05 | 0.05 | 0.1 |
| A����Ӧ�ﵽƽ��ʱ��Y��ת����Ϊ75% |
| B���÷�Ӧ�ɱ�ʾΪX+3Y?2Z���ҳ����µ�ƽ�ⳣ��Ϊ1600 |
| C��������������ʱ������ѹǿ��ʹ����ƽ��������Z�ķ����ƶ�����ƽ�ⳣ�������� |
| D���������¶���ʹ�÷�Ӧ��ƽ�ⳣ����С��˵���÷�Ӧ������ӦΪ���ȷ�Ӧ |
�ö��Ե缫���1mol/L CuSO4��0.1mol/L Cu��NO3��2�Ļ��Һ100mL������������896mL����״��������ʱ���жϵ�Դ������Һ��Ϊ100mL������˵����ȷ���ǣ�������
| A����������7.04gͭ |
| B�������缫��ӦΪ��Cu2++2e-�TCu��2H++2e-�TH2�� |
| C�������缫��ӦΪ��4OH--4e-�T2H2O+O2�� |
| D����������Һ�м���ͭ����ܽ�7.04gͭ |

