��Ŀ����
����A��D�������±��е������γɵģ���Ϊ��ѧ��ѧ�������ʣ������������������Ӹ�һ�֣��������ʵ����Ӳ����ظ���
A���������ֽ⣬����ɫ��ӦΪ��ɫ������ɫ�ܲ���������B��C�мӰ�ˮ���г���������B�еij����������������У�������ܽ⣻��D��Һ����C��Һ�еõ���������������ʣ�
��1���ֱ�����������ʵĻ�ѧʽ B ��C ��D ��
��2��д��A�ֽ�Ļ�ѧ����ʽ�� ��
��3��������A��D��Ӧ�����ӷ���ʽ�� ��
��4��C��Һ����ɫ�� ��
| ������ | K+��Al3+��Fe2+��Ba2+ |
| ������ | OH-��Cl-��SO42-��HCO3- |
��1���ֱ�����������ʵĻ�ѧʽ B
��2��д��A�ֽ�Ļ�ѧ����ʽ��
��3��������A��D��Ӧ�����ӷ���ʽ��
��4��C��Һ����ɫ��
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
����������̼�����Ƶ������жϣ�̼�����μ����ֽ⣬A����ɫ��ӦΪ��ɫ������ɫ�ܲ���������AΪKHCO3��
��B��C�мӰ�ˮ���г�������Al3+��Fe2+���ɼ����B�еij����������������У�������ܽ⣬��B����Al3+��C�к���Fe2+����D�к���Ba2+����D��Һ����C��Һ�еõ���������������ʣ������ᱵ�����˼����ƶϵó���CΪFeSO4��BΪAlCl3��DΪBa��OH��2��AΪKHCO3���ݴ˽���С�⼴�ɣ�
��B��C�мӰ�ˮ���г�������Al3+��Fe2+���ɼ����B�еij����������������У�������ܽ⣬��B����Al3+��C�к���Fe2+����D�к���Ba2+����D��Һ����C��Һ�еõ���������������ʣ������ᱵ�����˼����ƶϵó���CΪFeSO4��BΪAlCl3��DΪBa��OH��2��AΪKHCO3���ݴ˽���С�⼴�ɣ�
���
�⣺����̼�����Ƶ������жϣ�̼�����μ����ֽ⣬A����ɫ��ӦΪ��ɫ������ɫ�ܲ���������AΪKHCO3��
��B��C�мӰ�ˮ���г�������Al3+��Fe2+���ɼ����B�еij����������������У�������ܽ⣬��B����Al3+��C�к���Fe2+����D�к���Ba2+����D��Һ����C��Һ�еõ���������������ʣ������ᱵ�����˼����ƶϵó���CΪFeSO4��BΪAlCl3��DΪBa��OH��2��AΪKHCO3��
��1�����ݷ�����֪��BΪAlCl3��CΪFeSO4��DΪBa��OH��2���ʴ�Ϊ��AlCl3��FeSO4��Ba��OH��2��
��2��KHCO3�ֽ����̼��ء�������̼��ˮ����ѧ��Ӧ����ʽΪ��2KHCO3
K2CO3+CO2��+H2O���ʴ�Ϊ��2KHCO3
K2CO3+CO2��+H2O��
��3��AΪKHCO3��DΪBa��OH��2��������KHCO3��Ba��OH��2��Ӧ����̼�ᱵ�������������غ�ˮ����ѧ��Ӧ����ʽΪ��KHCO3+Ba��OH��2=BaCO3��+KOH+H2O�����ӷ�Ӧ����ʽΪ��HCO 3-+Ba2++OH-=BaCO3��+H2O���ʴ�Ϊ��HCO 3-+Ba2++OH-=BaCO3��+H2O��
��4��Fe2+Ϊdz��ɫ���ʴ�Ϊ��dz��ɫ��
��B��C�мӰ�ˮ���г�������Al3+��Fe2+���ɼ����B�еij����������������У�������ܽ⣬��B����Al3+��C�к���Fe2+����D�к���Ba2+����D��Һ����C��Һ�еõ���������������ʣ������ᱵ�����˼����ƶϵó���CΪFeSO4��BΪAlCl3��DΪBa��OH��2��AΪKHCO3��
��1�����ݷ�����֪��BΪAlCl3��CΪFeSO4��DΪBa��OH��2���ʴ�Ϊ��AlCl3��FeSO4��Ba��OH��2��
��2��KHCO3�ֽ����̼��ء�������̼��ˮ����ѧ��Ӧ����ʽΪ��2KHCO3
| ||
| ||
��3��AΪKHCO3��DΪBa��OH��2��������KHCO3��Ba��OH��2��Ӧ����̼�ᱵ�������������غ�ˮ����ѧ��Ӧ����ʽΪ��KHCO3+Ba��OH��2=BaCO3��+KOH+H2O�����ӷ�Ӧ����ʽΪ��HCO 3-+Ba2++OH-=BaCO3��+H2O���ʴ�Ϊ��HCO 3-+Ba2++OH-=BaCO3��+H2O��
��4��Fe2+Ϊdz��ɫ���ʴ�Ϊ��dz��ɫ��
������������Ҫ������dz������ӵļ����Լ����ӹ������⣬���ڸ߿���Ƶ���⣬ע���ܽ���ɣ�

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
���и������ʵ���Һ�У��ڿɼ��ȵ������½��������ʼ����Ӧ����һһ�����һ���ǣ�������
| A��KHSO4��Na2CO3����NH4��2SO4��FeCl3 |
| B��K2CO3��MgCl2��Al2��SO4��3��KOH |
| C��NaCl��KCl��CuCl2��AgNO3 |
| D��NaOH����NH4��2SO4��Na2SO4��BaCl2 |
�ں��¡����ݵ������н��з�Ӧ��2HI?H2+I2������ӦΪ���ȷ�Ӧ������Ӧ���Ũ����0.1mol/L����0.06mol/L��Ҫ20s����ô��0.06mol/L����0.036mol/L����ʱ��Ϊ��������
| A������10 s |
| B������12 s |
| C������12 s |
| D����12 s |

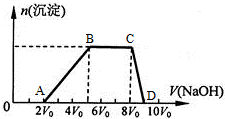

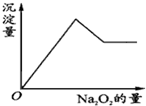 ��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ��
��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ��