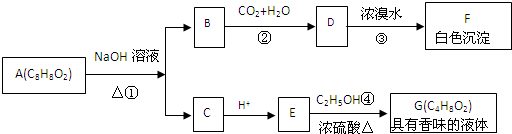
��Ŀ����
���л���A����Է�������Ϊ74���������ͼ�ֱ����£�

��1��A�Ľṹ��ʽΪ�� ��
����Է�������������100���л���B������������Ʒ�Ӧ������ɫ���壬������̼���Ʒ�Ӧ������ɫ���壬������ʹ������Ȼ�̼��Һ��ɫ�� B��ȫȼ��ֻ����CO2��H2O���������京��Ԫ�ص���������Ϊ37.21%�����˴Ź����ⷢ��B���������£�

��2��B�Ľṹ��ʽΪ�� ��
�������dz��õĺϳɸ߷��Ӳ��ϣ���������F��G������ij�������з�Ӧ�õ���

��3��д����Ӧ�ٵĻ�ѧ����ʽ�� ��
��4��д����Ӧ�ڵĻ�ѧ����ʽ�� ��
��5��д����Ӧ�۵Ļ�ѧ����ʽ�� ��

��1��A�Ľṹ��ʽΪ��
����Է�������������100���л���B������������Ʒ�Ӧ������ɫ���壬������̼���Ʒ�Ӧ������ɫ���壬������ʹ������Ȼ�̼��Һ��ɫ�� B��ȫȼ��ֻ����CO2��H2O���������京��Ԫ�ص���������Ϊ37.21%�����˴Ź����ⷢ��B���������£�

��2��B�Ľṹ��ʽΪ��
�������dz��õĺϳɸ߷��Ӳ��ϣ���������F��G������ij�������з�Ӧ�õ���

��3��д����Ӧ�ٵĻ�ѧ����ʽ��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��
��5��д����Ӧ�۵Ļ�ѧ����ʽ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1����������ͼ��֪���л�������ԭ������Ϊ74���������ͼ��ʾ���ڶԳƵļ����ԳƵ��Ǽ����Ѽ��ɵ÷��ӽṹ��
���л����������Na��Ӧ������ɫ���壬˵������-OH��-COOH��������̼���Ʒ�Ӧ������ɫ���壬˵������-COOH��������ʹ������Ȼ�̼��Һ��ɫ��˵�����в����ͼ�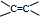 ��-C��C-�����ݺ���Ԫ�ص���������Ϊ37.21%����Է�������������100��ȷ����������ԭ�Ӹ���������ȷ���л������Է���������ȷ���л���ķ���ʽ�����ݺ˴Ź�������ȷ����������ԭ�����ͣ���Ͽ��ܵĹ����ţ�ȷ���л���ṹ��
��-C��C-�����ݺ���Ԫ�ص���������Ϊ37.21%����Է�������������100��ȷ����������ԭ�Ӹ���������ȷ���л������Է���������ȷ���л���ķ���ʽ�����ݺ˴Ź�������ȷ����������ԭ�����ͣ���Ͽ��ܵĹ����ţ�ȷ���л���ṹ��
��C3H6�ܷ����Ӿ۷�Ӧ��Ӧ����C=C��ΪCH3CH=CH2����FΪ ��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ
��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ ��Cȩ������������������D������DΪ
��Cȩ������������������D������DΪ ��D�ʻ�����������E������EΪ
��D�ʻ�����������E������EΪ ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH���Դ˽����⣮
OH���Դ˽����⣮
���л����������Na��Ӧ������ɫ���壬˵������-OH��-COOH��������̼���Ʒ�Ӧ������ɫ���壬˵������-COOH��������ʹ������Ȼ�̼��Һ��ɫ��˵�����в����ͼ�
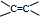 ��-C��C-�����ݺ���Ԫ�ص���������Ϊ37.21%����Է�������������100��ȷ����������ԭ�Ӹ���������ȷ���л������Է���������ȷ���л���ķ���ʽ�����ݺ˴Ź�������ȷ����������ԭ�����ͣ���Ͽ��ܵĹ����ţ�ȷ���л���ṹ��
��-C��C-�����ݺ���Ԫ�ص���������Ϊ37.21%����Է�������������100��ȷ����������ԭ�Ӹ���������ȷ���л������Է���������ȷ���л���ķ���ʽ�����ݺ˴Ź�������ȷ����������ԭ�����ͣ���Ͽ��ܵĹ����ţ�ȷ���л���ṹ����C3H6�ܷ����Ӿ۷�Ӧ��Ӧ����C=C��ΪCH3CH=CH2����FΪ
 ��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ
��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ ��Cȩ������������������D������DΪ
��Cȩ������������������D������DΪ ��D�ʻ�����������E������EΪ
��D�ʻ�����������E������EΪ ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH���Դ˽����⣮
OH���Դ˽����⣮���
�⣺������ͼ��֪���л�������ԭ������Ϊ74���������ͼ��ʾ���ڶԳƵļ����ԳƵ��Ǽ����Ѽ��ɵ÷��ӵĽṹ��ʽΪ��CH3CH2OCH2CH3��
�ʴ�Ϊ��CH3CH2OCH2CH3��
��2�����л�������Ԫ�ص���������Ϊ37.21%����Է�������������100�����Է�������ԭ����ĿN��O����
=2.3�����л�������̼���Ʒ�Ӧ������ɫ���壬˵������-COOH������̼ԭ����Ϊ2����֪�����ԭ������Ϊ
��=86������ΪB��ȫȼ��ֻ����CO2��H2O������ֻ����̼���⡢������Ԫ�أ����仯ѧʽΪ��CaHb-COOH����B����Է�������Ϊ86��֪��CaHb-��ʽ��Ϊ12a+b=41����a=1����b=29�����������ṹ����a=2����b=17�����������ṹ����a=3����b=5�������ʸ��л���ķ���ʽΪC4H6O2��B����ʽΪC4H6O2�������Ͷ�Ϊ
=2������һ���Ȼ�����B�ܹ�ʹ������Ȼ�̼��Һ��ɫ�����Ի�Ӧ��1��̼̼˫�����Ӻ˴Ź����ⷢ��B�����ֲ�ͬ�����µ���ԭ�ӣ�������ṹ��ʽΪ��CH2=C��CH3��-COOH��
�ʴ�Ϊ��CH2=C��CH3��-COOH��
��C3H6�ܷ����Ӿ۷�Ӧ��Ӧ����C=C��ΪCH3CH=CH2����FΪ ��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ
��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ ��Cȩ������������������D������DΪ
��Cȩ������������������D������DΪ ��D�ʻ�����������E������EΪ
��D�ʻ�����������E������EΪ ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH��
OH��
��3��AΪCH3CHBrCH2Br��±�����ڼ��������·���ˮ�ⷴӦ���ɴ������Է�ӦΪ��CH3CHBrCH2Br+2NaOH
CH3CHOHCH2OH+2NaBr��
�ʴ�Ϊ��CH3CHBrCH2Br+2NaOH
CH3CHOHCH2OH+2NaBr��
��4��C3H6ΪCH3CH=CH2���ܷ����Ӿ۷�Ӧ�����Է�Ӧ��ΪnCH3CH=CH2
 ��
��
�ʴ�Ϊ��nCH3CH=CH2
 ��
��
��5��EΪ ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH�����Է�Ӧ��Ϊ��nCH3-CHOH-COOH��H
OH�����Է�Ӧ��Ϊ��nCH3-CHOH-COOH��H OH+��n-1��H2O��
OH+��n-1��H2O��
�ʴ�Ϊ��nCH3-CHOH-COOH��H OH+��n-1��H2O��
OH+��n-1��H2O��
�ʴ�Ϊ��CH3CH2OCH2CH3��
��2�����л�������Ԫ�ص���������Ϊ37.21%����Է�������������100�����Է�������ԭ����ĿN��O����
| 100��37.21% |
| 16 |
| 16��2 |
| 37.21% |
| 4��2+2-6 |
| 2 |
�ʴ�Ϊ��CH2=C��CH3��-COOH��
��C3H6�ܷ����Ӿ۷�Ӧ��Ӧ����C=C��ΪCH3CH=CH2����FΪ
 ��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ
��CH3CH=CH2���嵥�ʷ�Ӧ������AΪCH3CHBrCH2Br��A�ڼ���������ˮ�⣬����BΪCH3CHOHCH2OH��������������C������CΪ ��Cȩ������������������D������DΪ
��Cȩ������������������D������DΪ ��D�ʻ�����������E������EΪ
��D�ʻ�����������E������EΪ ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH��
OH����3��AΪCH3CHBrCH2Br��±�����ڼ��������·���ˮ�ⷴӦ���ɴ������Է�ӦΪ��CH3CHBrCH2Br+2NaOH
| �� |
�ʴ�Ϊ��CH3CHBrCH2Br+2NaOH
| �� |
��4��C3H6ΪCH3CH=CH2���ܷ����Ӿ۷�Ӧ�����Է�Ӧ��ΪnCH3CH=CH2
| ���� |
 ��
���ʴ�Ϊ��nCH3CH=CH2
| ���� |
 ��
����5��EΪ
 ��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H
��E�����ǻ����Ȼ���G��E�������۷�Ӧ���ɣ�H OH�����Է�Ӧ��Ϊ��nCH3-CHOH-COOH��H
OH�����Է�Ӧ��Ϊ��nCH3-CHOH-COOH��H OH+��n-1��H2O��
OH+��n-1��H2O���ʴ�Ϊ��nCH3-CHOH-COOH��H
 OH+��n-1��H2O��
OH+��n-1��H2O��
������������Ҫ������ͨ������ͼ�ͺ������ͼ�ж����ʵĽṹ��ʽ���л���ĺϳɵ�֪ʶ����Ϥ�����л���Ľṹ�����ʣ����ݼ���ȷ���л��������DZ�����ѵ㣬�ʼĺϳ�ע�����C3H6�ܷ����Ӿ۷�ӦΪCH3CH=CH2��������ʵ����������Ʒ������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
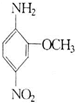 2-����-5�����������׳ƺ�ɫ��B����Ҫ��������ά֯���Ⱦɫ��Ҳ�����ƽ�ơ���졢�ڵ��л����ϣ���ṹ��ʽ��ͼ��ʾ��������ʽ���ɫ��B��ͬ���Ұ�����-NH2����������-NO2��ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ��������ɫ��B������Ϊ��������
2-����-5�����������׳ƺ�ɫ��B����Ҫ��������ά֯���Ⱦɫ��Ҳ�����ƽ�ơ���졢�ڵ��л����ϣ���ṹ��ʽ��ͼ��ʾ��������ʽ���ɫ��B��ͬ���Ұ�����-NH2����������-NO2��ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ��������ɫ��B������Ϊ��������| A��4�� | B��6�� | C��8�� | D��10�� |
���й����ȼ��仯�����˵����ȷ���ǣ�������
| A������Cl2��ȼ�գ������������� |
| B��HClO�����ᣬ����NaCIO��������� |
| C��Cl2ͨ��H2S������Һ�г��ֻ��ǣ�˵�������ԣ�Cl2��S |
| D��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
MOHǿ����Һ�͵��������Ũ�ȵ�HA�����Ϻ���Һ���й�����Ũ�ȵıȽ���ȷ���ǣ�������
| A��c��M+����c��OH-����c��A-����c��H+�� |
| B��c��M+����c��A-����c��H+����c��OH-�� |
| C��c��M+����c��A-����c��OH-����c��H+�� |
| D��c��M+����c��H+����c��A-����c��OH-�� |
�ں��º��ݵ�ij�ܱ������У��������л�ѧƽ�⣺C��s��+H2O��g��
CO��g��+H2��g�������������в���˵���������淴Ӧ�Ѵﵽ��ѧƽ��״̬���ǣ�������
| ���� |
| A����ϵ��ѹǿ���ٷ����仯 |
| B������nmolCO��ͬʱ����n��molH2 |
| C��v����CO��=v����H2O�� |
| D��1molH-H�����ѵ�ͬʱ2molH-O������ |
��������pH=13��ǿ����pH=2��ǿ����Һ��ϣ����û��Һ��pH=11����ǿ����ǿ��������Ϊ��������
| A��9��1 | B��1��11 |
| C��1��9 | D��11��1 |