��Ŀ����
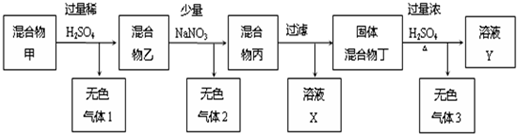
�������������մɲ��ϡ����ӹ�ҵ������ҽҩ�ȷ����й�����Ӧ��ǰ��������ͨ��������茶����ȷֽ�õ���[��֪��������茶���Ļ�ѧʽΪAl2��NH4��2��SO4��4?24H2O����Է�������Ϊ906]
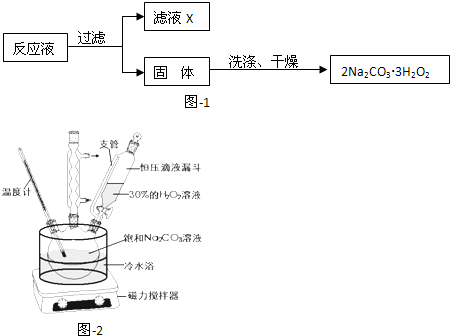
��1���Ʊ�������茶����ʵ���������£�
�ټ������������С����ˡ��������Ƿ������ʵ�鷽���� ��
�����������У������롱�������IJ�������Ϊ�� �� �����ˡ�ϴ�ӡ����
��2��ȡ4.53g ������茶�����ȷֽ⣬����ʣ��0.51g Al2O3���壮���ȹ����У������������¶ȵı仯��ͼ��ʾ��
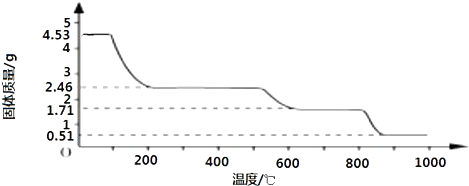
��ͨ������ȷ��400��ʱʣ�����ɷֵĻ�ѧʽ��д��������̣���
��1���Ʊ�������茶����ʵ���������£�

�ټ������������С����ˡ��������Ƿ������ʵ�鷽����
�����������У������롱�������IJ�������Ϊ��
��2��ȡ4.53g ������茶�����ȷֽ⣬����ʣ��0.51g Al2O3���壮���ȹ����У������������¶ȵı仯��ͼ��ʾ��

��ͨ������ȷ��400��ʱʣ�����ɷֵĻ�ѧʽ��д��������̣���
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
�������ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺���Һ��Ҫ������泥����������������Ϸ�Ӧ������������Һ���ٽ�����狀���������Һ��Ϸ�Ӧ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���������յõ�������茶��壬
��1�����ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺����Һ�п��ܺ���Fe3+����KSCN��Һ�����Ƿ���Fe3+��
���ɹ������̿�֪�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
��2�����㾧����ˮ�����������ݹ�����������㾧����ʣ��ᾧˮ����������������ʣ�������n[��NH4��Al��SO4��2]��n��H2O�����ݴ���д��ѧʽ��
��1�����ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺����Һ�п��ܺ���Fe3+����KSCN��Һ�����Ƿ���Fe3+��
���ɹ������̿�֪�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
��2�����㾧����ˮ�����������ݹ�����������㾧����ʣ��ᾧˮ����������������ʣ�������n[��NH4��Al��SO4��2]��n��H2O�����ݴ���д��ѧʽ��
���
�⣺�ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺���Һ��Ҫ������泥����������������Ϸ�Ӧ������������Һ���ٽ�����狀���������Һ��Ϸ�Ӧ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���������յõ�������茶��壻
��1�����ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺����Һ�п��ܺ���Fe3+��ȡ������Һ���Թ��У��Ӽ���KSCN��Һ������Һ�����ɫ���������ѳ�����
�ʴ�Ϊ��ȡ������Һ���Թ��У��Ӽ���KSCN��Һ������Һ�����ɫ���������ѳ�����
���ɹ������̿�֪�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ��ʴ�Ϊ������Ũ������ȴ�ᾧ��
��2��ȡ4.53g������茶�������ʵ���Ϊ
=0.005mol��
4.53g������茶�����ˮ������Ϊ0.005mol��24��18g/mol=2.16g��
����400��ʱ�����������١�m=4.53g-2.46g=2.07g��2.16g
ʣ������нᾧˮ�����ʵ���Ϊ
=0.005mol��
ʣ�������n[��NH4��2Al2��SO4��4]��n��H2O��=0.005mol��0.005mol=1��1��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4?H2O��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4?H2O��
��1�����ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˺����Һ�п��ܺ���Fe3+��ȡ������Һ���Թ��У��Ӽ���KSCN��Һ������Һ�����ɫ���������ѳ�����
�ʴ�Ϊ��ȡ������Һ���Թ��У��Ӽ���KSCN��Һ������Һ�����ɫ���������ѳ�����
���ɹ������̿�֪�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ��ʴ�Ϊ������Ũ������ȴ�ᾧ��
��2��ȡ4.53g������茶�������ʵ���Ϊ
| 4.53g |
| 906g/mol |
4.53g������茶�����ˮ������Ϊ0.005mol��24��18g/mol=2.16g��
����400��ʱ�����������١�m=4.53g-2.46g=2.07g��2.16g
ʣ������нᾧˮ�����ʵ���Ϊ
| 2.16g-2.07g |
| 18g/mol |
ʣ�������n[��NH4��2Al2��SO4��4]��n��H2O��=0.005mol��0.005mol=1��1��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4?H2O��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4?H2O��
���������⿼��þ�����仯��������ʣ��Թ����������⡢���û�ѧ�������ˮ�⡢���ʵķ����ᴿ�����Ӽ��顢��ѧ����ȣ��ۺ��Խϴ��ѶȽϸߣ���Ҫѧ���߱���ʵ�Ļ���������֪ʶ������������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
����ʵ����������۾���ȷ���ǣ�������
| A����Һ����ʱ����Һ©�����²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| B��ij�����м���ϡ���ᣬ������ɫ��ζ����ʹ����ʯ��ˮ����ǵ����壬֤���ù�����һ������CO32- |
| C���������Һ���ȼ���BaCl2��Һ��������ɫ�������ټ����������ᣬ�������ܽ⣬֤����Һ��һ������SO42- |
| D����Ũ��������һ�����ʵ���Ũ�ȵ�ϡ����ʱ����Ũ����ϡ�ͺ�Ӧ��ȴ�����º���ת�Ƶ�����ƿ�� |
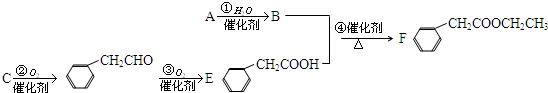
ij�л���Ļ�ѧʽΪC5H10O�����ܷ���������Ӧ���������H2�ӳ����ò���Ľṹ��ʽ�������ǣ�������
| A��CH3��CH2��3CH2OH |
| B��CH3CH2CH��CH3��CH2OH |
| C����CH3��2CHCH2CH2OH |
| D����CH3CH2��2CHOH |
 ��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����ͲӦ����
��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����ͲӦ����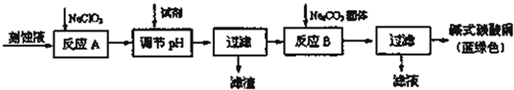
 ��������
��������