��Ŀ����
17�� �㽶ˮ��Ҫ����������ܼ���ϡ�ͼ�������Ҫ�ɷ�����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
�㽶ˮ��Ҫ����������ܼ���ϡ�ͼ�������Ҫ�ɷ�����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
| �������� | ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ��� |
| ���촼 | 88 | 0.8123 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |
ʵ�鲽�裺
��A�м���4.4g���촼��6.0g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A�����Ⱥͼг�װ���ԣ�������50min����ӦҺ�������º����Һ©���У���������ˮ������̼��������Һϴ������ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�������������3.9g��
�ش��������⣺
��1������ʵ������з������Ǽ�����Ƭ����Ӧ��ȡ�Ĵ�ʩ��ֹͣ���ȣ�����ӦҺ��ȴ��������Ƭ��
��2����ϴ�Ӳ����еڶ�����ˮϴ����ҪĿ����ϴ��̼�����ƣ�
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��ֲ�ƷӦ�ӷ�Һ©�����ϣ���ϡ����¡����ڷ������
��4��ʵ���м���������ˮMgSO4��Ŀ���Ǹ��
��5����ʵ��IJ�����c�����ţ���
a��30% b��40% c��60% d��90%
��6���ڽ����������ʱ������ʵ��IJ���ƫ�ߣ������ԭ����δ��140����ռ���֣����ռ�����δ��Ӧ�����촼����
���� ��1������ʵ������з������Ǽ�����Ƭ����ɵ���Һ��ɽ��������¼��룬Ӧע���ֹʵ���¹ʵķ�����
��2���ڶ���ˮϴ����ҪĿ���dz�ȥ��Ʒ�в�����̼�����ƣ�
��3���������������ܶȱ�ˮС��Ӧ��Һ���ϲ㣻
��4��������ˮ����þ�ܹ�����������������������ˮ�֣��������ã�
��5���ȼ������������촼�����ʵ�����Ȼ���жϹ�����������ݲ�����������������������������������ʵ�����������ʵ������ȡ��������������������ʣ�
��6������130��㿪ʼ�ռ���ִ�ʱ�������к������촼�����ռ�������δ��Ӧ�����촼�����»�õ���������������ƫ��
��� �⣺��1�����Ƭ�Ķ�ṹ���ڿ��Է�ֹ�ڼ��ȹ����в����������������Ƭʱ��Ҫ���Ѽ��ȵ���Һ��ȴ���ټ��룬
�ʴ�Ϊ��ֹͣ���ȣ�����ӦҺ��ȴ��������Ƭ��
��2����Ӧ�����ҺҪ�������ϴ�ӣ���ϴ�Ӳ����У���һ��ϴ�ӵ���ҪĿ���dz�ȥ�ִ�������ʹ����һ���б���̼��������Һ�ȿ��Գ�ȥδϴ���Ĵ��ᣬҲ���Խ��������ܽ�ȣ�����һ��ϴ�Ӻ����ɵ����л���̼�����ƣ����Եڶ���ˮϴ����ҪĿ���dz�ȥ��Ʒ�в�����̼�����ƣ�
�ʴ�Ϊ��ϴ��̼�����ƣ�
��3���������������ܶȱ�ˮС��Ӧ��Һ���ϲ㣬���ô��Ͽڵ������ʴ�Ϊ���ϣ�
��4��ʵ���м���������ˮ����þ��Ŀ������������������ˮ�֣�������и��
�ʴ�Ϊ�����
��5����������ʵ���Ϊ��n=$\frac{6.0g}{60g/mol}$=0.1mol�����촼�����ʵ���Ϊ��n=$\frac{4.4g}{88g/mol}$=0.05mol��������������촼�ǰ���1��1���з�Ӧ���������������������������������Ҫ�������촼�����ʵ������㣬������������0.05mol������������ʵ�������ɵ����������������ʵ���Ϊ��$\frac{3.9g}{130g/mol}$=0.03mol������ʵ���������������IJ���Ϊ��$\frac{0.03mol}{0.05mol}$��100%=60%��
�ʴ�Ϊ��c��
��6���ڽ����������ʱ������130��㿪ʼ�ռ���ִ�ʱ�������к������촼�����ռ�������δ��Ӧ�����촼����˻ᵼ�²���ƫ�ߣ�
�ʴ�Ϊ��δ��140����ռ���֣����ռ�����δ��Ӧ�����촼����
���� �����ۺϿ������ʵ��Ʊ���Ϊ�߿��������ͣ���Ŀ�漰���������Ĺ����밲װ�������ķ��롢�ᴿ�����ʵ���ȡ��ҩƷ��ѡ��ʹ�á����ʲ��ʵļ����֪ʶ����Ŀ�ѶȽϴ������漰�������ϴ�֪ʶ��϶࣬���������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� �����̿��Ʊ�������ص���Ҫ��Ӧ���£�
�����̿��Ʊ�������ص���Ҫ��Ӧ���£���������3MnO2+KClO3+6KOH$\frac{\underline{\;����\;}}{\;}$3K2MnO4+KCl+3H2O
�����绯3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3
������ʵ��ܽ�ȣ�293K�����±���
| K2CO3 | KHCO3 | K2SO4 | KMnO4 | |
| �ܽ��/g | 111 | 33.7 | 11.1 | 6.34 |
a�������� b�������� c�������� d��������
��2������ʱ�����������ԭ��������K2SO4�ܽ��С���ή�Ͳ�Ʒ�Ĵ��ȣ������������ԭ����������л�ԭ�ԣ��ᱻ���������Ͳ�Ʒ������
��3�����õ�ⷨҲ����ʵ��K2MnO4��ת����2K2MnO4+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������ΪK2MnO4�е���Ԫ�ؿ�����ȫת����KMnO4�У���������ʣ�
��4�������Ƶζ�������������ش��Ȳ������£�
���ȡ0.80g ���ҵĸ�����ز�Ʒ�����50mL��Һ��
��ȷ��ȡ0.2014g�����Ѻ�ɵ�Na2C2O4��������ƿ�У�������������ˮʹ���ܽ⣬�ټ������������ữ��
��ƿ����Һ���ȵ�75��80�棬�����â������Ƶĸ��������Һ�ζ����յ㣮��¼���ĸ��������Һ�����������ó���Ʒ���ȣ�
�ٵζ������з�Ӧ�����ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ڴﵽ�ζ��յ�ı�־Ϊ��ƿ����Һͻ��Ϊdz��ɫ�Ұ�����ڲ���ɫ��
�ۼ����¶ȴ���90�棬���ֲ��ᷢ���ֽ⣬�ᵼ�²�ò�Ʒ����ƫ�ߣ����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ܽ�һ�������������Һ���ữ�IJ�������Һ��ϣ���÷�ӦҺ��Mn2+��Ũ���淴Ӧʱ��t�ı仯����ͼ����ԭ�����Ϊ���ɵ�Mn2+������������Mn2+Ũ�����ӣ���Ӧ����Խ��Խ�죮
| A�� | 6.02��1023�ǰ����ӵ������Ľ���ֵ | |
| B�� | �����ӵ³������������ʵ�����1 mol | |
| C�� | 1 mol 12Cԭ�ӵ�����Ϊ12 g | |
| D�� | �����Ħ��������98 g |

��1��������Ϊ���ˣ��������õ��IJ����������ձ�����Һ©����
��2���������������ҪĿ���dz�ȥ���ʡ�����ͭԪ�أ�
��3��С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2��OH��2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu��OH��2��Cu4��OH��6SO4��
��֪Cu��OH��2��Cu2��OH��2CO3��Cu4��OH��6SO4��������ˮ����������ֽ��¶�����Ϊ80�桢200�桢300�森
���ʵ���������Һ�ɷ֣���ɱ������ݣ�
��ѡ�Լ���2 mol•L-1 HCl��1 mol•L-1 H2SO4��0.1 mol•L-1 NaOH��0.1 mol•L-1BaCl2������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У��������2mol/L��������Һ��������ٵμӼ���0.1mol/L�Ȼ�����Һ | �а�ɫ��������˵������Һ�л���Cu4��OH��6SO4�� |
| ����2����ȡ��������Һ���Թ��У����Թܷ���װ�з�ˮ��С�ձ���ˮԡ����һ��ʱ��ȡ���Թܹ۲� | �Թ����к�ɫ�������ɣ�˵������Һ�л���Cu��OH��2�� |
�±���25��ʱijЩ�ε�Ũ�Ȼ�������
| ��ѧʽ | CaSO4 | Ag2SO4 | PbSO4 |
| Ksp | 4.9��10-5 | 1.2��10-5 | 1.6��10-8 |
A���ձ� B����Ͳ C������ƿ D����ƿ E��������
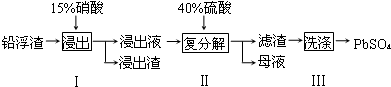
��2������I��NO����������Һ�к������Ľ���������Ϊ Pb2+��д��Pb�μӷ�Ӧ�Ļ�ѧ����ʽ3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��Ϊ��ֹAg���ܽ������Һ������I����ʱӦע����������������ʹPb����ʣ�࣮
��3��ĸҺ��ѭ�������ڲ���I����������Ҫ��HNO3����һ�����ʻ�ѧʽ������ĸҺ�в����� SO42-���࣬ѭ������ʱ���ܳ��ֵ������ǽ���ʱ����Pb2+����PbSO4��������ų�������PbSO4�IJ��ʣ�
��4����PbSO4 ��Ʒ���е�������CaSO4������Pb��NO3��2��Һ���ϴ�ӣ��Եõ�������PbSO4��
��5��Ǧ���طŵ�ʱ�����ĵ缫��ӦʽΪPbO2+2e-+4H++SO42-=PbSO4+2H2O�������Ǧ��������Դ��ⱥ��ʳ��ˮ��ȡCl2����֪ijǦ������������Һ�����Ϊ0.80L�����ǰ������ҺŨ��Ϊ4.50mol•L-1�����Ƶ�4.48LCl2ʱ���ڱ�״���£����������ϵ�������������Һ��Ũ��Ϊ��������ǰ��������Һ��������䣩4 mol•L-1��

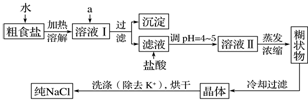
 ������������ɫ����ˮ����ζ��Һ�壬�е�77.1�棬ʵ����ij����ȡ���ñ�����14.3mL��95%�Ҵ�23mL�����õ�ŨH2SO4������Na2CO3��Һ�Լ��������Ҵ���ϳ�����������Ȼ�����Һ����Ҫ����װ����ͼ��ʾ��ʵ�鲽���ǣ�������A������ƿ��ע�������Ҵ������õ�ŨH2SO4��ҡ�ȣ��ٽ�ʣ�µ������Ҵ��ͱ�����ע���Һ©�����ã���ʱ��Һ©�����Ҵ������������ʵ���֮��ԼΪ7��5���ڼ�����ԡ�������¶���135�桫145��֮�䣻�۽���Һ©���е�Һ�建������������ƿ����ڼ����ٶ�ʹ���������ٶ�������ٶȴ�����ȣ�ֱ��������ɣ��ܱ�����ԡ�¶�һ��ʱ�䣬��������Һ�������ֹͣ���ȣ���ȡ��B����ƿ����һ��������Na2CO3��Һ����������εؼӵ����Һ��ӱ�ҡ����ֱ�������ݲ���Ϊֹ�����ݵ�Һ�������Һ����ȥˮ�㣻�߽�����CaCl2��Һ�����������뵽��Һ©���У�ҡ��һ��ʱ�侲�ã��ų�ˮ�㣨��Һ�������Һ©����õ����dz����ᴿ������������Ʒ����Ȼ���������м�����ˮ�����ƣ���
������������ɫ����ˮ����ζ��Һ�壬�е�77.1�棬ʵ����ij����ȡ���ñ�����14.3mL��95%�Ҵ�23mL�����õ�ŨH2SO4������Na2CO3��Һ�Լ��������Ҵ���ϳ�����������Ȼ�����Һ����Ҫ����װ����ͼ��ʾ��ʵ�鲽���ǣ�������A������ƿ��ע�������Ҵ������õ�ŨH2SO4��ҡ�ȣ��ٽ�ʣ�µ������Ҵ��ͱ�����ע���Һ©�����ã���ʱ��Һ©�����Ҵ������������ʵ���֮��ԼΪ7��5���ڼ�����ԡ�������¶���135�桫145��֮�䣻�۽���Һ©���е�Һ�建������������ƿ����ڼ����ٶ�ʹ���������ٶ�������ٶȴ�����ȣ�ֱ��������ɣ��ܱ�����ԡ�¶�һ��ʱ�䣬��������Һ�������ֹͣ���ȣ���ȡ��B����ƿ����һ��������Na2CO3��Һ����������εؼӵ����Һ��ӱ�ҡ����ֱ�������ݲ���Ϊֹ�����ݵ�Һ�������Һ����ȥˮ�㣻�߽�����CaCl2��Һ�����������뵽��Һ©���У�ҡ��һ��ʱ�侲�ã��ų�ˮ�㣨��Һ�������Һ©����õ����dz����ᴿ������������Ʒ����Ȼ���������м�����ˮ�����ƣ���