��Ŀ����
3����ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷��� FeSO4•7H2O ������1������98% 1.84g/cm3��Ũ������������28%��������Һ����Ũ������ˮ�������ԼΪ1��4.6��
��2�����̷����ȷֽ��IJ���Ϊ������Ԫ�صĹ���A��SO2��SO3��H2O���ش��������⣺
�̷����ȷֽ����Ԫ�صĻ��ϼ��ڷֽ�ǰ���Ƿ����˱仯�ǣ���ǡ��������������̷��ֽ��������SO2����Ԫ�ػ��ϼ۽��ͣ�����Ԫ�ػ��ϼ����ߣ�
��3��Ϊ�ⶨij�����ڿ������̷���Ʒ��Fe2+�������ʣ�ijͬѧ�������ʵ�飺ȡһ��������Ʒȫ���ܽ���ϡ�����У�Ȼ�����5.00g���۳�ַ�Ӧ���ռ���224mL����״�������壬ʣ���������Ϊ3.88g����÷�Ӧ�����Һ��Fe2+�����ʵ���Ϊ0.14mol�������Ʒ��Fe2+���ӵ�������Ϊ16.7%��
��4�����������[��NH4��2SO4•FeSO4•6H2O]���׳�Ī���Σ������̷��ȶ�����������ԭ�ζ������г���������Fe2+�ı���Һ����ȡ0.4g Cu2S��CuS�Ļ������������Һ����40mL 0.150mol/L KMnO4��Һ������������Ӧ���£�
8MnO4-+5Cu2S+44H+��10Cu2++5SO2+8Mn2++22H2O
6MnO4-+5CuS+28H+��5Cu2++5SO2+6Mn2++14H2O
��Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����V mL 0.2mol/L ��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ����֪��MnO4-+5Fe2++8H+��Mn2++5Fe3++4H2O
��V��ȡֵ��ΧΪ25��V��50��
����V=35���Լ���������CuS������������
���� ��1����������������غ���������ˮ������ȣ�
��2���̷��� FeSO4•7H2O ������Ļ��ϼ�Ϊ+6�ۣ���Ӧ�����˶��������ϼ۽����ˣ�����������ԭ��Ӧ��������֪��һ����Ԫ�صĻ��ϼ����ߣ��ݴ��жϣ�
��3���ȸ��������������������������Ҫ�������������ɵĶ��������ӵ����ʵ������ٸ����ܼ��ٵ�����������ȥ����������Ҫ����������Ϊ�����������ӷ�Ӧ��Ҫ�������������������������ӷ�Ӧ���������������������ӵ����ʵ��������ɶ��������ӵ����ʵ������ܶ��������ӵ����ʵ�����ȥ�������������ӵ����ʵ�����Ϊԭ��Һ�ж��������ӵ����ʵ��������������ӵ����ʵ�����ԭ��Һ�ж��������Ӻ�����������֮�͵ı�ֵ��Ϊ�����ʣ�
��4���ٲ��ü���������������ﷴӦ��Ҫ�ĸ�����ص����ʵ�����ʣ��ĸ�������루NH4��2Fe��SO4��2��Һ��Ӧ������Ҫ�ģ�NH4��2Fe��SO4��2��Һ�������
���ȸ��ݣ�NH4��2Fe��SO4��2�����ʵ����������䷴Ӧ�ĸ�����ص����ʵ��������������������������������������������������Ҫ�ĸ�����ص����ʵ�����Ȼ����ʽ��������������������������������ʽ���㼴�ɣ�
��� �⣺��1����Ũ��������ΪxmL��ˮ�����ΪymL��ϡ��ǰ��������������䣬
����$\frac{1.84x��98%}{1.84x+1.0y}$�����x��y=1��4.6��
�ʴ�Ϊ��4.6��
��2���̷��� FeSO4•7H2O ������Ļ��ϼ�Ϊ+6�ۣ���Ӧ�����˶��������ϼ۽����ˣ�����������ԭ��Ӧ��������֪��һ����Ԫ�صĻ��ϼ����ߣ����Կ����ж�����Ԫ�صĻ��ϼ��ڷ�Ӧ�����������ˣ�
�ʴ�Ϊ���ǣ� �̷��ֽ��������SO2����Ԫ�ػ��ϼ۽��ͣ�����Ԫ�ػ��ϼ����ߣ�
��3�������ᷴӦ����������Ҫ����������Ϊx�����ɵ��������������ʵ���Ϊy��
Fe+2H+=Fe 2++H2 ��
56g 1mol 22.4L
x y 0.224L
���x=0.56g��y=0.01mol��
��Fe3+��Ӧ��Ҫ����������Ϊ5.00g-3.88g-0.56g=0.56g��
������Ӧ��Ҫ�����������ӵ����ʵ���Ϊm�����ɵĶ��������ӵ����ʵ���Ϊn��
Fe+2Fe3+=3Fe2+
56g 2mol 3mol
0.56g m n
���m=0.02mol��n=0.03mol��
����ԭ����Һ�ж��������ӵ����ʵ���Ϊ0.14mol-0.01mol-0.03mol=0.1mol��
����������Ϊ$\frac{0.02mol}{0.1mol+0.02mol}$��
�ʴ�Ϊ��16.7%��
��4���ٸ�����ص����ʵ���Ϊ��0.040L��0.150mol/L=0.006mol��
�����������ȫ��ΪCu2S����Ҫ������ص����ʵ���Ϊx��
8MnO4-+5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O
8 5
x $\frac{0.4g}{160g}$
x=0.004mol
ʣ��ĸ�����ص����ʵ���Ϊ0.006mol-0.004mol=0.002mol��
0.002mol������غͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.002mol 1��10-3VL��0.2mol/L
���V=50��
�����������ȫ��ΪCuS����Ҫ������ص����ʵ���Ϊy��
6MnO4-+5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O
6 5
y $\frac{0.4g}{96g}$
y=0.005mol
ʣ��ĸ�����ص����ʵ���Ϊ0.006mol-0.005mol=0.001mol��
0.001mol������غͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.001mol 1��10-3VL��0.2mol/L
���V=25��
�ʴ�Ϊ��25��V��50��
����V=35����35mL��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��Ҫ������ص����ʵ���Ϊ��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.014mol 0.035L��0.2mol/L
���Ը�����ص����ʵ���Ϊ0.0014mol��
����������ﷴӦ�ĸ�����ص����ʵ���Ϊ0.006mol-0.0014mol=0.0046mol��
��Cu2S������Ϊxg��CuS������Ϊyg��x+y=0.40g��
8MnO4-+5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O
8mol 800g
0.01xmol xg
6MnO4-+5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O
6mol 480g
0.0125ymol yg
$\left\{\begin{array}{l}{x+y=0.4}\\{0.01xmol+0.0125ymol=0}\end{array}\right.$
���$\left\{\begin{array}{l}{x=0.16\\;}\\{y=0.24}\end{array}\right.$
��������CuS����������=$\frac{0.24g}{0.4g}$��
�𣺻������CuS����������Ϊ60%��
���� ���⿼���˸��ݷ���ʽ�����йؼ��㣬�ѶȽϴ�ע����������ʱ���ü��������ݼ����������ȡֵ��Χ��

| A�� | 2��5-�������� | B�� | 2��3-�������� | ||
| C�� | 2��3��4-�������� | D�� | 2��2��3��3-�ļ����� |
| A�� | �����ڳ�ȥCO2�е�SO2 | B�� | ��������ȡ20.00mL Na0H��Һ | ||
| C�� | �������ж�װ�õ������� | D�� | �����ڽ�����ȼ�ճɻ� |
| A�� | ij��Һ��Na0H��Һ���ȣ�����ʹʪ���ʯ����ֽ�������壬˵��ԭ��Һ�д���NH${\;}_{4}^{+}$ | |
| B�� | ij��Һ�м���AgN03��Һʱ��������ɫ������˵��ԭ��Һ�к���Cl- | |
| C�� | �ò�˿պȡij��Һ�ھƾ��ƻ���������ʱ������ʻ�ɫ��˵��ԭ��Һ�к��н����� | |
| D�� | ij��Һ�м���BaCl2��Һʱ��������ɫ������ԭ��Һ�п��ܴ���Ag+��SO42-��CO32- |
| A�� | ��������ʵ���Ũ����0.5mol•L-1 | B�� | ����������Һ�������250mL | ||
| C�� | ��������ʵ���֮��Ϊ2��1 | D�� | ���������֮��Ϊ1��2 |

| A�� | a��b�Ƚϣ�bʹ���˴��� | B�� | a��b�Ƚϣ�b�¶ȸ��� | ||
| C�� | a��b�Ƚϣ�b��ѹǿ���� | D�� | a��b�Ƚϣ�b��Ӧ���ʸ��� |
2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g����H=-92.3KJ/mol
��Ӧ�����У�SO2��O2��SO3�����ʵ�����mol���ı仯���±���0��4minʱ����������ѹǿΪ0.1MPa����
| ʱ��min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | w.9 |
| n��SO2�� | 2.00 | 1.92 | 1.84 | 1.76 | 1.76 | 1.64 | 1.52 | 1.40 | 1.40 | 1.40 |
| n��O2�� | 1.00 | 0.96 | 0.92 | 0.88 | 0.88 | 0.82 | 0.76 | 0.70 | 0.70 | 0.70 |
| n��SO3�� | w0 | 0.08 | 0.16 | 0.24 | 0.24 | 0.36 | 0.48 | 0.60 | 0.60 | 0.60 |
��1��3��4min��7��9minʱ�Σ���Ӧ����ƽ��״̬��
��2����5minʱ����ͬʱ������ʺ�ת������������������ı���������������ѹǿ��ƽ�����������ƶ���������������桱������
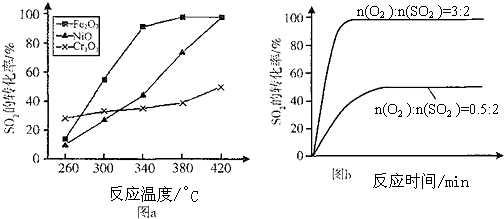
��3������������ͬ��������ͬʱ��S02��ת�����淴Ӧ�¶ȵı仯��ͼa��260��Cr2O3 ����Fe203��Ni0��Cr203����������Ӧ������죮Fe2O3��Ni0����������ʹS02��ת���ʴﵽ��ߣ������Ǽ۸����أ�ѡ��Fe203�� ��Ҫ�ŵ���Fe2O3������ʱ������Խϵ��¶ȿɻ�ýϸ�SO2��ת���ʣ�
��4������С����3800C Fe203������ʱ���о��˲�ͬͶ�ϱ�n��O2����n��SO2����S02ת���ʵ�Ӱ�죬�����ͼb�����ڴ������ͼ�л���n��O2����n��SO2��=1��2ʱ��SO2ת���ʵ�Ԥ�ڱ仯���ߣ�