��Ŀ����
9��A��B��C���Ƕ�����Ԫ�أ�ԭ��������������B�ǵؿ��к�������Ԫ�أ�������AxBy�ж�����ʽ���еĿ��Ե������ꣻ���ӻ�����CmBnҲ�в�ͬ��ʽ������m��n������2��1��1��1��������ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�ɴ�С��˳���ǣ�C��B��A | |
| B�� | AxBy��A�Ļ��ϼ۲�����Ϊ+1�� | |
| C�� | C��B�γɵĻ���������ˮ���ܵõ�����B | |
| D�� | A��B��Ԫ�ص���̬�⻯��������Ӧ |
���� A��B��C���Ƕ�����Ԫ�أ�ԭ��������������B�ǵؿ��к�������Ԫ�أ���BΪOԪ�أ�������AxBy�ж�����ʽ���еĿ��Ե������꣬��AΪNԪ�أ����ӻ�����CmBnҲ�в�ͬ��ʽ������m��n������2��1��1��1����CΪNa���ݴ˽��
��� �⣺A��B��C���Ƕ�����Ԫ�أ�ԭ��������������B�ǵؿ��к�������Ԫ�أ���BΪOԪ�أ�������AxBy�ж�����ʽ���еĿ��Ե������꣬��AΪNԪ�أ����ӻ�����CmBnҲ�в�ͬ��ʽ������m��n������2��1��1��1����CΪNa��
A��ͬ�����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ԭ�Ӱ뾶Na��N��O����A����
B��N2O��NԪ�ػ��ϼ�Ϊ+1����B����
C��C��B�γɵĻ������������ơ��������ƣ�����������ˮ��Ӧ��������������������ˮ��Ӧ�����������ƣ���C����
D��������ˮ��Ӧ����һˮ�ϰ�����D��ȷ��
��ѡD��
���� ���⿼��Ԫ�ػ������ƶϣ���Ҫѧ���������ճ���Ԫ�ػ�������ɼ����ʣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
4�����������к�����һ������Ӧ���ǣ�������
| A�� | Ba��OH��2 | B�� | CaCO3 | C�� | Na2SO4 | D�� | CH3CH2OH |
14�����ڷ�ӦA+3B=2C�������ʾ�Ϊ���壩�����и����ݱ�ʾ��ͬ�����µķ�Ӧ���ʣ����з�Ӧ���������ǣ�������
| A�� | v��A��=0.01mol/��L•S�� | B�� | v��B��=0.02mol/��L•S�� | C�� | v��B��=0.60mol/��L•S�� | D�� | v��C��=1.0mol/��L•S�� |
1����ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�����������

��1��A����ȡCO2��װ�ã�����a�����Ʒ�Һ©��д��A�з�����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2����
��2����д���пո����ڴ������д��
��3��д�����������������̼��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2=2Na2CO3+O2���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��1
��4�����ʵ���ҽ�1.5mol����������3mol̼�����ƹ����Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų��������ʺ���ȴ�������Ĺ���������Na2CO3��

��1��A����ȡCO2��װ�ã�����a�����Ʒ�Һ©��д��A�з�����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2����
��2����д���пո����ڴ������д��
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | |
| C | Na2O2 | ��CO2��ˮ������Ӧ������O2 |
| D | �� |
��4�����ʵ���ҽ�1.5mol����������3mol̼�����ƹ����Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų��������ʺ���ȴ�������Ĺ���������Na2CO3��
19�����и���Ԫ�����ʵݱ����������ǣ�������
| A�� | Li��Be��Bԭ������������������ | |
| B�� | P��S��Cl��������������� | |
| C�� | N��O��F�õ����������� | |
| D�� | Li��Na��K��Rb�Ľ�����������ǿ |
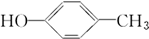
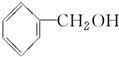
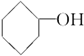

 ���ߵĵ���ʽΪ
���ߵĵ���ʽΪ ��
��