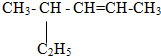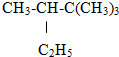��Ŀ����
ijѧ�����ʵ�鷽��֤�������к���K+��Al3+��SO42-�����������ɱ���
| ʵ�鲽�� | ʵ������ | ���ۼ����� |
| ��1��ȡ��������������������ˮ�У�����֧�Թֱܷ�ȡ������Һ�������ò�˿պȡ�Թ�1����Һ���� | �� | ���ۣ�����K+�� |
| ��2�����Թ�2�м������������ữ���μ����� | �а�ɫ������ | ���ۣ����� ���ͣ� ��д���ӷ���ʽ�� |
| ��3�����Թ�3����μ�������NaOH��Һ���ӱ��� | ���ۣ����� ���ͣ� ��д���ӷ���ʽ�� |
���㣺�������ӵļ��鷽��
ר�⣺���ʼ��������
��������1������K+����ͨ����ɫ�ܲ����۲���ɫ��������ɫΪ��ɫ��
��2������Al3+����ȡ����������Һ����������μ�������������Һ���۲�����Ƿ��ܽ⣻
��3������SO42-�����ȼ������ᣬ���������ټ����Ȼ�����Һ�۲��Ƿ��г������ɣ�
��2������Al3+����ȡ����������Һ����������μ�������������Һ���۲�����Ƿ��ܽ⣻
��3������SO42-�����ȼ������ᣬ���������ټ����Ȼ�����Һ�۲��Ƿ��г������ɣ�
���
�⣺��1������K+����ͨ����ɫ�ܲ����۲���ɫ��Ӧ�������������Ϊ���ò�˿պȡ����������Һ�����ھƾ��ƻ��������գ������ܲ����۲쵽�������ɫ��
�ʴ�Ϊ����ɫ����ɫ�ܲ������ϣ�
��2������SO42-�����ȼ������ᣬ�ų��������ӣ��������������ټ���BaCl2��Һ�۲��Ƿ��г������ɣ������ɰ�ɫ��������֤������SO42-��
�ʴ�Ϊ��BaCl2��SO42-��Ba2++SO42-=BaSO4����
��3������Al3+����ȡ����������Һ����������μ�������������Һ���۲�����Ƿ��ܽ⣬�����а�ɫ����������������ܽ⣬��֤������Al3+��
�ʴ�Ϊ���տ�ʼ�а�ɫ������Ȼ���ɫ������ʧ��Al3+��Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+H2O����Al3++4OH-=AlO2-+2H2O����
�ʴ�Ϊ����ɫ����ɫ�ܲ������ϣ�
��2������SO42-�����ȼ������ᣬ�ų��������ӣ��������������ټ���BaCl2��Һ�۲��Ƿ��г������ɣ������ɰ�ɫ��������֤������SO42-��
�ʴ�Ϊ��BaCl2��SO42-��Ba2++SO42-=BaSO4����
��3������Al3+����ȡ����������Һ����������μ�������������Һ���۲�����Ƿ��ܽ⣬�����а�ɫ����������������ܽ⣬��֤������Al3+��
�ʴ�Ϊ���տ�ʼ�а�ɫ������Ȼ���ɫ������ʧ��Al3+��Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+H2O����Al3++4OH-=AlO2-+2H2O����
���������⿼�����ʵļ��������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע�����ճ������ӵĻ�ѧ���ʣ���ȷ�������ӵļ��鷽������Ƽ��鷽��ʱ�������ų��������ӣ�ȷ�����鷽���������ԣ�

��ϰ��ϵ�д�
�����Ŀ
 �ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g����H��0���ڷ�Ӧ�����У��淴Ӧ���ʵı仯��ͼ��ʾ������˵����ȷ���ǣ�������
�ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g����H��0���ڷ�Ӧ�����У��淴Ӧ���ʵı仯��ͼ��ʾ������˵����ȷ���ǣ�������| A��t1ʱһ���������������� |
| B��t2ʱʹ���˴��� |
| C��t3ʱ������ѹǿ |
| D��t4ʱһ���ǽ������¶� |