��Ŀ����
3��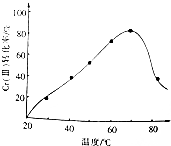 ��ҵ������ˮ�Ĵ���ԭ���ǽ�Cr2O72-ת��ΪCr3+���ٽ�Cr3+ת��ΪCr��OH��3�������������Ṥҵ�����е�SO2����������ˮ���ȳ��������Դ���Է��ηϣ����ܽ�Լ�����ɱ���
��ҵ������ˮ�Ĵ���ԭ���ǽ�Cr2O72-ת��ΪCr3+���ٽ�Cr3+ת��ΪCr��OH��3�������������Ṥҵ�����е�SO2����������ˮ���ȳ��������Դ���Է��ηϣ����ܽ�Լ�����ɱ�����1����ҵ�ϴ���100LCr2O72-����Ϊ108mg•L-1�ĺ�����ˮ��������Ҫ3.36L����״����SO2��
��2����֪��Ksp[Cr��OH��3]=1��10-30�������£���ȥ��SO2��ԭ������Һ�е�Cr3+��ʹ��Ũ��С��1��10-4mol•L-1�����������ҺpH��6��
��3�����۸�Cr������˫��ˮ��Ӧ�����ںϳɸ��ƣ�PbCrO4�������������������䣬���ڷ�Ӧ�¶ȣ����췴Ӧ�¶ȶ�Cr����ת���ʵ�Ӱ�죨��ͼ��ʾ�����¶ȳ���70��ʱ��Cr����ת�����½���ԭ���ǽϸ��¶���˫��ˮ�ֽ⣮
��4�������£����ᣨH2C2O4��Ҳ�ܽ�Cr2O72-ת��ΪCr3+����ѧʽΪAl2Fe��SO4��4��ij���Σ�ë��״���ڿ������ܱ��������Ը÷�Ӧ���д����ã�Ϊȷ��һƿ���÷��εĻ�ѧ�ɷ֣�ѧϰС���������ʵ�飺ȡһ�������ķ�����Ʒ����������ϡ�����У�����Һ��Ϊ���ȷݣ�����һ��������KMnO4��Һ��ַ�Ӧ����Ӧ��MnO4-����ԭΪMn2+��������Ũ��Ϊ0.4000mol•L-1��KMnO4��Һ20.00mL������һ����Һ�м�������ϡ��ˮ���ڿ������Ȳ�����ʹ֮��ַ�Ӧ�����������ٱ仯����ˣ�������ϴ�Ӳ�������պ��������9.100g��������ĩ��
ͨ��������������룬�Ʋ�þ��÷��εĿ��ܻ�ѧ��ɣ����������������Ʋ����ɣ�
���� ��1��100LCr2O72-����Ϊ108mg•L-1�ĺ�����ˮ�к�Cr2O72-���ʵ�����=100L��108mg•L-1��=108mg=0.108g�����ʵ���n��Cr2O72-��=$\frac{0.108g}{216g/mol}$=0.0005mol����϶��������Cr2O72-��Ӧ�Ķ�����ϵ���㣬Cr2O72-��2Cr3+��6e-��SO2��SO42-��2e-��Cr2O72-��3SO2��6e-��
��2�������ܶȻ���������Ksp[Cr��OH��3]=1��10-30�������£���ȥ��SO2��ԭ������Һ�е�Cr3+��ʹ��Ũ��С��1��10-4mol•L-1����Ksp[Cr��OH��3]=c��Cr3+��c3��OH-��=1��10-30��c��OH-��=$\root{3}{\frac{1��1{0}^{-30}}{1��1{0}^{-4}}}$=10-8.67mol/L��
��3�����۸�Cr������˫��ˮ��Ӧ�����ںϳɸ��ƣ�PbCrO4������������������ԭ��Ӧ����Ӧ�ﵽƽ��״̬�ﵽ���ת���ʣ��¶����߹�������ֽ�ƽ��������У�
��4��n��KMnO4��=0.4000mol/L��20ml��10-3L/ml=8.000��10-3mol��
n��Fe2+��=5��8.000��10-3mol=0.04000mol�����ݷ��εĻ�ѧʽ��֪�ھ��õķ�����n��Al3+��2��[0.04000mol+n��Fe3+��]�����백ˮ���г��ĸ�������ĩ�У�
n��Al2O3��=0.04000mol+n��Fe3+��
n��Fe2O3��=$\frac{1}{2}$[0.04000mol+n��Fe3+��]
102g/mol��[0.04000mol+n��Fe3+��]+160g/mol��$\frac{1}{2}$��[0.04000mol+n��Fe3+��]=9.10g
n��Fe3+��=001000mol
n��Al3+��=2��[0.04000mol+n��Fe3+��]=0.1000mol
n��SO42-��=2n��Al3+��=0.2000mol
���õķ����е��������������������3n��Al3+��+2n��Fe2+��+3n��Fe3+��=0.4100mol
����֪�������������������2n��SO42-��=0.4000mol��0.4100mol�����ڷ����ڿ����б������������л����ܴ���0.01000molOH-��0.00500molCO32-���ݴ���д��ѧʽ��
��� �⣺��1��100LCr2O72-����Ϊ108mg•L-1�ĺ�����ˮ�к�Cr2O72-���ʵ�����=100L��108mg•L-1��=10800mg=10.8g�����ʵ���n��Cr2O72-��=$\frac{10.8g}{216g/mol}$=0.05mol����϶��������Cr2O72-��Ӧ�Ķ�����ϵ���㣬Cr2O72-��2Cr3+��6e-��SO2��SO42-��2e-��
Cr2O72-��3SO2��6e-��
1 3
0.05mol n
n=0.15mol��
��״�������=22.4L/mol��0.15mol=3.36L��
�ʴ�Ϊ��3.36��
��2��Ksp[Cr��OH��3]=1��10-30�������£���ȥ��SO2��ԭ������Һ�е�Cr3+��ʹ��Ũ��С��1��10-4mol•L-1����Ksp[Cr��OH��3]=c��Cr3+��c3��OH-��=1��10-30��c��OH-��=$\root{3}{\frac{1��1{0}^{-30}}{1��1{0}^{-4}}}$=10-8.67mol/L��c��H+��=$\frac{1{0}^{-14}}{1{0}^{-8.67}}$=10-5.33mol/L��PH=5.33��Cr3+ʹ��Ũ��С��1��10-4mol•L-1���������ҺpH��6��
�ʴ�Ϊ����6��
��3�����۸�Cr������˫��ˮ��Ӧ�����ںϳɸ��ƣ�PbCrO4������������������ԭ��Ӧ��ͼ�������֪��Ӧ�ﵽƽ��״̬�ﵽ���ת���ʣ��¶����߳���70��ʱ��������ֽ⣬ʹƽ��������У�Cr����ת�����½���
�ʴ�Ϊ���ϸ��¶���˫��ˮ�ֽ⣻
��4��n��KMnO4��=0.4000mol/L��20ml��10-3L/ml=8.000��10-3mol��
n��Fe2+��=5��8.000��10-3mol=0.04000mol�����ݷ��εĻ�ѧʽ��֪�ھ��õķ�����n��Al3+��2��[0.04000mol+n��Fe3+��]�����백ˮ���г��ĸ�������ĩ�У�
n��Al2O3��=0.04000mol+n��Fe3+��
n��Fe2O3��=$\frac{1}{2}$[0.04000mol+n��Fe3+��]
102g/mol��[0.04000mol+n��Fe3+��]+160g/mol��$\frac{1}{2}$��[0.04000mol+n��Fe3+��]=9.10g
n��Fe3+��=001000mol
n��Al3+��=2��[0.04000mol+n��Fe3+��]=0.1000mol
n��SO42-��=2n��Al3+��=0.2000mol
���õķ����е��������������������3n��Al3+��+2n��Fe2+��+3n��Fe3+��=0.4100mol
����֪�������������������2n��SO42-��=0.4000mol��0.4100mol�����ڷ����ڿ����б������������л����ܴ���0.01000molOH-��0.00500molCO32-��
�÷��εĿ������Ϊn��Al3+����[n��Fe2+��+n��Fe3+��]��n��OH-����n��SO42-��=0.1��0.05��0.01��0.2=10��5��1��20����ѧʽΪ��Al10Fe5��OH����SO4��20��
n��Al3+����[n��Fe2+��+n��Fe3+��]��n��CO32-����n��SO42-��=0.1��0.05��0.005��0.2=20��10��1��40����ѧʽΪAl20Fe10��CO3����SO4��40��
��n��KMnO4��=0.4000mol/L��20ml��10-3L/ml=8.000��10-3mol��
n��Fe2+��=5��8.000��10-3mol=0.04000mol�����ݷ��εĻ�ѧʽ��֪�ھ��õķ�����n��Al3+��2��[0.04000mol+n��Fe3+��]�����백ˮ���г��ĸ�������ĩ�У�
n��Al2O3��=0.04000mol+n��Fe3+��
n��Fe2O3��=$\frac{1}{2}$[0.04000mol+n��Fe3+��]
102g/mol��[0.04000mol+n��Fe3+��]+160g/mol��$\frac{1}{2}$��[0.04000mol+n��Fe3+��]=9.10g
n��Fe3+��=001000mol
n��Al3+��=2��[0.04000mol+n��Fe3+��]=0.1000mol
n��SO42-��=2n��Al3+��=0.2000mol
���õķ����е��������������������3n��Al3+��+2n��Fe2+��+3n��Fe3+��=0.4100mol
����֪�������������������2n��SO42-��=0.4000mol��0.4100mol�����ڷ����ڿ����б������������л����ܴ���0.01000molOH-��0.00500molCO32-��
�÷��εĿ������Ϊn��Al3+����[n��Fe2+��+n��Fe3+��]��n��OH-����n��SO42-��=0.1��0.05��0.01��0.2=10��5��1��20����ѧʽΪ��Al10Fe5��OH����SO4��20��
n��Al3+����[n��Fe2+��+n��Fe3+��]��n��CO32-����n��SO42-��=0.1��0.05��0.005��0.2=20��10��1��40����ѧʽΪAl20Fe10��CO3����SO4��40��
�þ��÷��εĿ��ܻ�ѧ���ΪAl10Fe5��OH����SO4��20��Al20Fe10��CO3����SO4��40��
���� ���⿼����������ɵ�ʵ��̽�����ܶȻ��������㡢��ѧʽ����Ӧ�á�������ԭ��Ӧ��������ķ����жϣ����ջ����ǽ���ؼ�����Ŀ�ѶȽϴ�

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�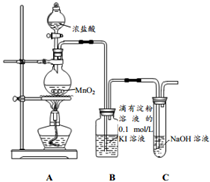 ̽��������KI��Һ�ķ�Ӧ��ij����С������ͼװ�ý���ʵ�飨�г���������ȥ���������Ѽ��飩
̽��������KI��Һ�ķ�Ӧ��ij����С������ͼװ�ý���ʵ�飨�г���������ȥ���������Ѽ��飩| ʵ����� | ʵ������ |
| ��A�з�Һ©�����������²���Ũ���ᣬ���� | װ��B����Һ����ɫ�������ɫ��ȥ����Һ��dz��ɫ |
��2��װ��C��NaOH�����������ն�����������ֹ��Ⱦ���������з�����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
��3����֤��������������ǿ�ڵ��ʵ��������Bװ����ͨ��������Һ������
��4��Ϊ̽��B����Һ��ɫ�仯��ԭ��С��ͬѧ�������ϲ�����ʵ�飮
�������ϣ�
I2��I-����Һ�лᷢ����Ӧ��I2+I-?I3-��I3-���ػ�ɫ�������۱�����I2�ɱ�����������ΪICl2-����ɫ����ICl4-����ɫ�����������ӿɼ�����������IO3-����ɫ����
����ʵ�飺
| ʵ����� | ʵ������ |
| ��ȡ��Ӧ��B�е���Һ4mL�ֳ����ȷݣ���һ�ݵ���1�ε�ˮ���ڶ��ݵ��������Һ | ��һ����Һ��ɫ �ڶ�����Һ��ɫû�б仯 |
| ��I2����KI��Һ�����Ƶõ���Ũ��Ϊ0.1mol/L����Һ��ȡ������Һ2mL���μ�1�ε�����Һ����ͨ������ | �ӵ��ۺ���Һ������ͨ��������ɫ��ȥ����Һ��dz��ɫ |
| �����������Һ����ͨ������ | ��Һ������Ϊ��ɫ |
��д��ICl2-��ˮ��Һ����������Ӧ����IO3-�����ӷ���ʽICl2-+2Cl2+3H2O=IO3-+6Cl-+6H+��
��������ʵ����ƶ�B����Һ��ɫ���dz��ɫ��ԭ����I-�ȱ�����������I2��I2�ֱ�����������ICl2-����ɫ����ICl4-����ɫ��������ϳ�dz��ɫ��Һ��
| A�� | ��ȥ��Ȳ�л��е�����H2S���壺����CuSO4��Һϴ�� | |
| B�� | ��ȥ��ȩ�л��е�����������������м���������ϡNaOH��Һ��Ȼ���Һ | |
| C�� | ��ȥ���������ı��ӣ����������м���������Ũ��ˮ������ | |
| D�� | ��ȥ�屽�л��е�����Һ�壺���������м���������ϡNaOH��Һ��Ȼ���Һ |
| A�� | 1molNH4Cl�����й��ۼ�����Ϊ5NA | |
| B�� | 16gO2��O3�Ļ�����к���NA����ԭ�� | |
| C�� | ��װ�д������ܱ���������3molH2��1molN2����ַ�Ӧ���2NA�������� | |
| D�� | 18gˮ��H2O������8NA������ |
| A�� | �ǽ���Ԫ�ع��ɵĵ�����һ�����ڹ��ۼ� | |
| B�� | �ɲ�ͬ��ԭ�Ӽ��γɵĹ��ۼ���һ���Ǽ��Լ� | |
| C�� | ���й��ۼ��Ļ����ﲻһ���ǹ��ۻ����� | |
| D�� | ��ѧ�����ѣ���һ��������ѧ�仯 |
| A�� | ú��������Һ����Ϊ��ѧ�仯����ʯ�͵ij�ѹ����ͼ�ѹ������������仯 | |
| B�� | ��ɫ��ѧ�ĺ��ľ������û�ѧԭ�����մ�����ҵ������Ⱦ�ﲢ����ת��Ϊ�������� | |
| C�� | ����ʯ�͵��ѻ�����������͵IJ��������� | |
| D�� | ����±ˮŨ�������������������ˮ��������ȡ�� |
| Ũ ʱ�� �� �¶� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 1 | 800�� | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| 2 | 800�� | C2 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 800�� | C3 | 0.92 | 0.75 | 0.63 | 0.60 | 0.60 | 0.60 |
| 4 | 820�� | 1.0 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 |
��1����ʵ��1�У���Ӧ��10��20minʱ���ڣ���Ӧ��A��ƽ������Ϊ0.013mol/��L•min����
��2����ʵ��2�У�A�ij�ʼŨ��C2=1.0mol/L����Ӧ��20minA��Ũ�ȾͲ��ٷ����仯���������Ʋ�ʵ��2�������������Ǽ����˴�����
��3����ʵ��3�У�A�ij�ʼŨ��C3��1.0mol/L�����=��������
��4���Ƚ�ʵ��4��ʵ��1�����Ʋ�÷�Ӧ�����ȷ�Ӧ��ѡ����ȡ��������ȡ����������������¶ȣ�A��ƽ��Ũ�ȼ�С��˵�������¶�ƽ��������Ӧ�����ƶ���������Ӧ�����ȷ�Ӧ��
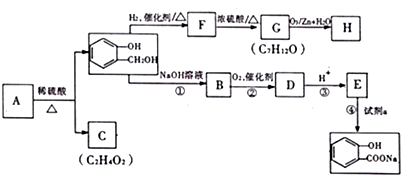
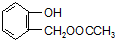 ��C���еĹ��������Ȼ�����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ��
��C���еĹ��������Ȼ�����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ��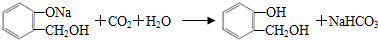 �����ʵ��Լ�aΪNaHCO3��Һ��
�����ʵ��Լ�aΪNaHCO3��Һ��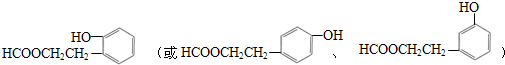 ��д��һ�ּ��ɣ���
��д��һ�ּ��ɣ���