��Ŀ����
����13�֣�ʵ������Ҫ90mL 2.0 mol��L��1��Na2CO3��Һ��������ˮ̼���Ʒ�δ���ƣ���ش��������⣺
��1������ͨ�����㣨Ҫ�м�����̣�����ȷ����ȡ g��ˮ̼���ơ�
��2�����������У������õ�����
A��50mL����ƿ�� B��100mL����ƿ�� C��������
D��100mL��Ͳ�� E��������ƽ�� F��ҩ��
��3����Ҫʵʩ���ƣ������������⣬��ȱ�������� ��
��4������ƿ��ʹ��ǰ������еIJ����� ��
��5�����ƹ��̼���Ϊ���¸���������ȷ�IJ���˳��Ϊ (����������)��
A����ȴ�����£� B��ϴ�Ӳ���Һ�� C����ȡ�� D���ܽ⣻
E��ҡ��װƿ�� F�����ݣ� G����Һ
��6�������ƹ����У����������Ũ���к�Ӱ��?
������ƿ������ˮϴ����û�ȵ������������Һ���ݣ���������Һ��Ũ�� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����
�� ת����Һʱ����С������Һ����ƿ�⣬��������Һ��Ũ��
�۶���ʱ�����ӿ̶��ߣ���������Һ��Ũ��
�����ڵμ�����ˮʱ�����������˿̶��ߣ���������Һ��Ũ�� ����ʱӦ��δ����� ��
��1�� 21.2 g��2�֣���m (Na2CO3) =" 0.1" L��2.0 mol/L��106g/mol=" 21.2" g
��2��A��D��2�֣� �� ��3���ձ���1�֣��� ��ͷ�ιܣ�1�֣���
��4�����ˮ���ԣ�1�֣��� ��5��C D A G B F E��1�֣���
��6������Ӱ�죨1�֣� ��ƫ�ͣ�1�֣� ��ƫ�ߣ�1�֣� ��ƫ�ͣ�1�֣�,ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�1�֣���
�������������������ʵ������û��90mL����ƿ����֮�����ӽ�����100ml������ƿ������Ӧ������100ml��Na2CO3��Һ��m(Na2CO3)="(c��V)��M=" 0.1 L��2.0 mol/L��106g/mol=" 21.2" g����2��Na2CO3�ǹ���ҩƷ��Ҫ��������ƽ��ҩ��������һ������������ҩƷ�����ձ����ܽ⣬���ò���������ٽ����ʵ��ܽ⣬Ȼ���ò�����������100mL����ƿ������ý�ͷ�ιܽ��ж��ݣ���ʹ�õ������Dz���ʹ�õ�������A��50mL����ƿ����D��100mL��Ͳ����3����Ҫʵʩ���ƣ������������⣬��ȱ���������ձ�����ͷ�ιܣ���4��������Һ�о�һ�ԣ�������Ũ����ͬ����������ƿ���Ƶ���ҺҪҡ�ȣ�����ʹ��ǰ������еIJ����Ǽ���������Ƿ����ã���5����ȡ���ܽ⣻��ȴ�����£���Һ��ϴ�Ӳ���Һ�����ݣ�ҡ��װƿ���ʴ�����C��D��A��G��B��F��E����6���� ����ƿ������ˮϴ����û�ȵ������������Һ���ݣ�������Һ��������䣬���ʵ����ʵ������䣬����������Һ��Ũ����Ӱ�죻�� ת����Һʱ����С������Һ����ƿ�⣬�����ʵ����ʵ���ƫ�٣�ʹ������Һ��Ũ��ƫ�ͣ��۶���ʱ�����ӿ̶��ߣ�����Һ������ڿ̶���һ�£���Һ�����ƫ�٣�ʹ������Һ��Ũ��ƫ�ߣ������ڵμ�����ˮʱ�����������˿̶��ߣ�����Һ�����ƫ��ʹ������Һ��Ũ��ƫ�ͣ���ʱ����������ϴ������ƿ���������ơ�
���㣺�������ʵ���Ũ�ȵ���Һ���Ƶ�֪ʶ����Ҫ����������ѡ�������衢�����������㡣

���в���������Ⱦ�����
| A��CO | B���ױ� | C����Ŷ� | D��ʳƷ�� |
14��)���ܶ�Ϊ1.84g/mL����������Ϊ98%��Ũ��������100 ml 3.0mol/L ϡ�����ʵ�鲽�����£��ټ�������Ũ�������� ����ȡһ�������Ũ���� ��ϡ�� ��ת��
��ϴ�� ���� ��ҡ��
��1������Ũ���������� ��
��2���ڢ۲�ʵ��IJ����� ��
��3���ڢ�ʵ��IJ����� ��
��4����������������Ƶ�ϡ����Ũ���к�Ӱ�죿������ĸ��дa��ƫ�� b��ƫС c����Ӱ�죩
| A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ������� |
| B������ƿ������ϴ�Ӻ������������ˮ |
| C�����ù����ձ���������δϴ�� |
| D����ȡŨ����ʱ������Ͳ�ϵĿ̶�ȡ��Ũ���� |
F������õ���Һ����������ˮϴ����δ�ɵ��Լ�ƿ�б���
��5����ʵ�����˷�ʱ��ĵط��ǽ�ϡ�ͺ��������ȴ�����£�Ϊ�˽�Լʱ�䣬�����еļ�
��ϡ������ȴ�ķ�����_______________________________________��
��16�֣���1��д����ͼ����Ţ١������������ƣ�
�� ���� ���� ���� ��
��2�������١����У�ʹ��ǰ�����©�� ������������ţ�
��3�������ˮ�еĵ�Ӧ�� ����װ����ţ����� �� ������
��4��������98%��Ũ����(�ܶ�Ϊ1��84g/cm3)���Ƴ�Ũ��Ϊ0��5 mol/L��ϡ����100mL��
�������������ձ������������ __________��__________��__________��
����ȡŨ��������Ϊ____________mL��
�����в�������������ҺŨ��ƫ�ߵ���
| A������ͲȡŨ����ʱ���� |
| B����Ũ���ᵹ����ϴ����Ͳ������ϴ��Һ�����ձ��� |
| C�����ձ���ϡ��Ũ���������ת�� |
| D������ʱ���� |
�ܱ�ʵ�����ʱ����ǽ�ϡ�ͺ��������ȴ�����£�Ϊ��ʡʱ�䣬�����еļӿ�ϡ������ȴ�ķ�����_______________________________________��
ʵ������NaOH��������250mL 1.25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������250mL 1.25mol/L��NaOH��Һ, Ӧ��ȡNaOH������ g��
��2������ƿ�ϱ��� ����ʹ��ǰ��һ�������� ��
��3����Һע������ƿǰ����ȴ�����£�������Ϊ_____________________________��
��4������ʱ�IJ��� ��
��5���������Ƶ���ҺŨ��ƫ�͵��� ��
| A������NaOHʱ��������������� |
| B��������ƿ��ת����Һʱ������Һ����������ƿ���� |
| C������ʱ���ӿ̶��� |
| D������ǰ������ƿ������������ˮ |
��14�֣�ʵ����
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������200 mL 1.0 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�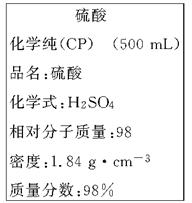
�ٲ�����������ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
��1����������ϡ����ʱ����ȱ�ٵ�������________________________(д��������)��
��2����ǩ��ʾŨ��������ʵ���Ũ��Ϊ___________________________
��3������200 mL 1.0 mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ_______mL������������С�����1λ������ȡ����ʱӦѡ��_______������Ͳ��
| A��10 mL | B��50 mL |
| C��100 mL | D��200 mL |
������Ͳȡ�������������Ũ���
������Ͳ�м�����������ˮ�����ò��������裻
��������ϡ�ͺ����Һת������ƿ�У�
��Ȼ������ˮ�ز�����ע������ƿֱ���̶��ߣ�
�ݰ�����ƿ�Ǹǽ������µߵ�ҡ�ȡ�
����Ϊ����ʵ���д���IJ�����______________________________������ţ�
��5�������ⶨ��ijͬѧ���Ƶ�ϡ����Ũ��ƫ�ߣ�����ܵ�ԭ����_______������ţ�
������Ͳ��ȡŨ����ʱ�����ӿ̶���
������ƿ������ˮϴ�Ӻ�δ������
��ϴ���ձ��ڱں�ϴ��Һ��ȥ
��ת����Һʱ��������������Һ����
�ݶ���ʱ����������ƿ�̶���
���ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶���
��8�֣�ʵ��������NaOH��������1.0 mol/L��NaOH��Һ240 mL��
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ����ڼ�����ܽ��ҡ�Ȣ���Һ��ϴ�Ӣ߶��ݢ���ȴ��ҡ��
����ȷ�IJ���˳��Ϊ____________________(�����)����ʵ������õ�������������
��ƽ��ҩ�ס����������ձ���_______�� ��
��2��ijͬѧ������NaOH������������������ƽ�����ձ���������������ƽƽ����״
̬��ͼ��ʾ���ձ���ʵ������Ϊ________g��Ҫ��ɱ�ʵ���ͬѧӦ����________g NaOH��
��3��ʹ������ƿǰ������е�һ�������� ��
��4�����ƹ����У����в���������������ҺŨ��ƫ�ߵ���
| A��ת����Һʱ����������Һ��������ƿ���� |
| B������ʱ���ӿ̶��� |
| C��δ��ȴ�����¾ͽ���Һ��������ƿ�в����� |
| D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�����ˮ���̶��� |
������˿��������ȼ�ա���˿�����˵����ȷ����
| A����˿ȼ�ղ������� | B����˿�����ǻ������� |
| C����˿ȼ�յIJ�����Fe2O3 | D���������Ҫ�ɷ���Fe3O4 |